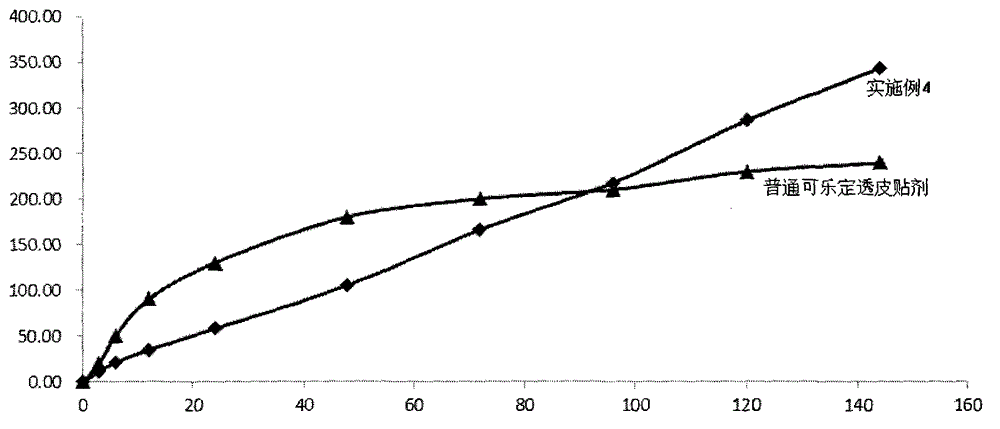
Transdermal drug delivery system
A technology of a transdermal drug delivery system and drug storehouse, which is applied in the fields of cardiovascular system diseases, antipyretics, drug combinations, etc., can solve the problems of complex use process, difficult manufacturing and high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
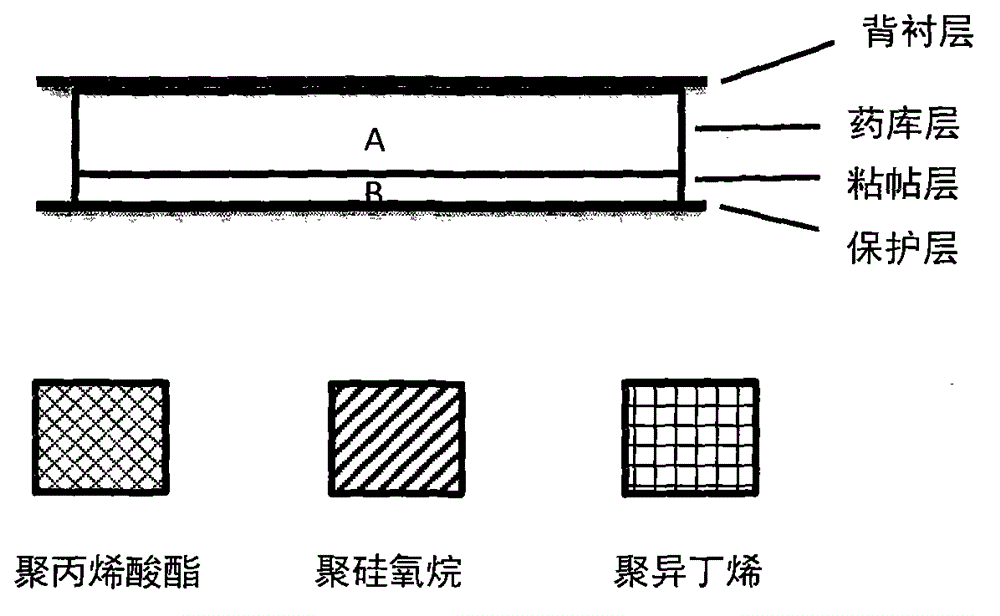
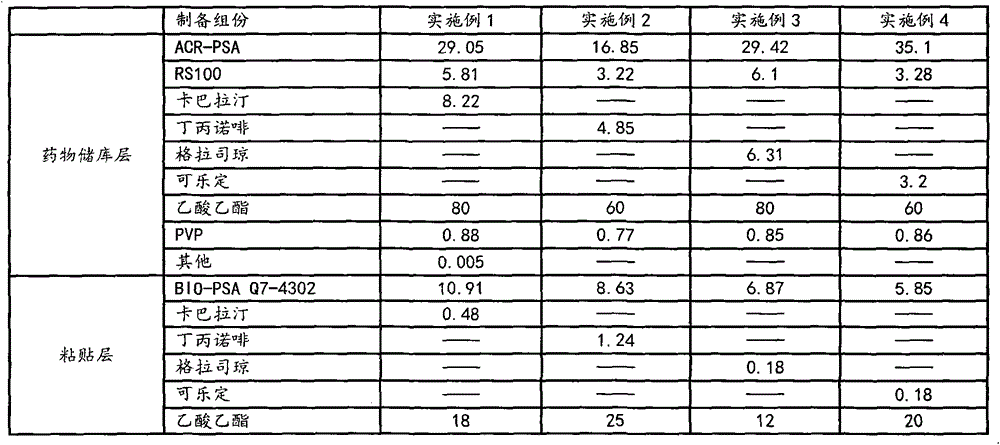
[0039] Each component was weighed according to the weight of each component of the drug storage layer and the adhesive layer in Example 1 in Table 1. After the components of the weighed drug storage layer are fully mixed and homogenized, the mixed colloid is coated on the backing layer material with a thickness of 0.65mm, and dried at 40°C in the dark for 2 hours and cooled to room temperature for later use; the weighed paste After the components of the layer are fully mixed and homogeneous, the mixed colloid is coated on the anti-adhesive protective layer material with a thickness of 0.30mm, and dried at 40°C in the dark for 2 hours and cooled to room temperature for later use; the two parts of the adhesive layer prepared above Composite with the drug storehouse layer and cut into 2.5cm by die 2 Specification embodiment 1 transdermal patch, with aluminum-plastic film airtight packaging, keep away from light for subsequent use.
Embodiment 2
[0041] Each component was weighed according to the weight of each component of the drug storage layer and the adhesive layer in Example 2 in Table 1. After the components of the weighed drug storage layer are fully mixed and homogenized, the mixed colloid is coated on the backing layer material with a thickness of 0.45mm, dried at 60°C for 2 hours and cooled to room temperature for later use; After the components are fully mixed and homogenized, the mixed colloid is coated on the anti-adhesive protective layer material with a thickness of 0.20mm, and dried at 60°C for 2 hours and cooled to room temperature for later use; The compound is punched and cut into 2.5cm by the mold 2 Specification Example 2 Transdermal patch, sealed and packaged with aluminum-plastic film, protected from light and stored for later use.
Embodiment 3
[0043] Each component was weighed according to the weight of each component of the drug storage layer and the adhesive layer in Example 3 in Table 1. After the components of the weighed drug storage layer are fully mixed and homogenized, the mixed colloid is coated on the backing layer material with a thickness of 0.70mm, dried at 60°C for 2 hours and cooled to room temperature for later use; After the components are fully mixed and homogenized, the mixed colloid is coated on the anti-adhesive protective layer material with a thickness of 0.20mm, and dried at 60°C for 2 hours and cooled to room temperature for later use; The compound is punched and cut into 2.5cm by the mold 2 Specification Example 3 The transdermal patch is sealed and packaged with aluminum-plastic film and stored away from light for subsequent use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com