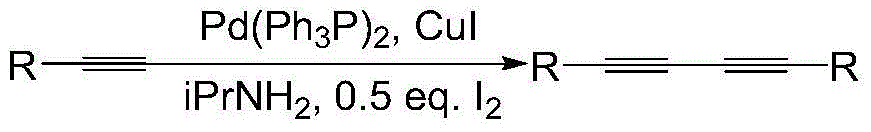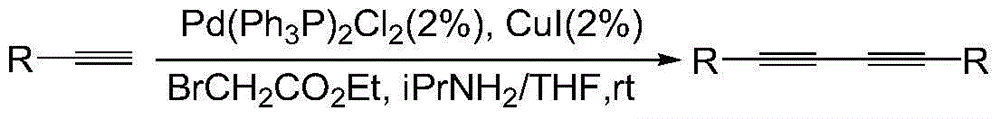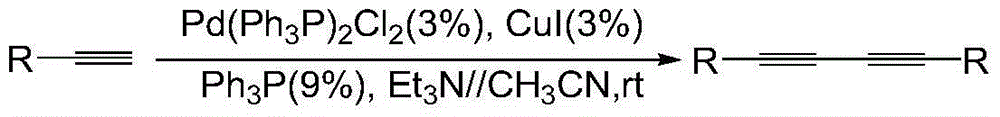Preparation method of 1,3-diacetylene catalytic system
A catalytic system, diacetylene technology, applied in the preparation of organic compounds, chemical instruments and methods, condensation hydrocarbons with dehydrogenated hydrocarbons, etc., can solve problems such as complex reaction processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Embodiment one: in reaction flask, add 2mmol phenylacetylene, 0.1mmolCuCl 2 ,0.2mmolK 2 CO 3 and 1 mL of DMF, and then reacted at 100° C. for 24 hours, and the reaction mixture was simply separated to obtain 0.196 g of 1,4-diphenyl-1,3-butadiyne, with a yield of 97%. Its NMR data are: 1 HNMR (CDCl 3 ,400MHz)(δ,ppm):7.32-7.40(m,6H,ArH),7.52-7.54(m,4H,ArH); 13 CNMR (CDCl 3 , 100 MHz) (δ, ppm) 132.5, 129.2, 128.4, 121.7, 81.5, 73.9; low resolution mass spectrum [LRMS (ESI+)] data: m / z = 202.08 (calculated value 202.25).
Embodiment 2
[0070] Embodiment two: in reaction bottle, add 2mmol4-butylphenylacetylene, 0.1mmolCuCl 2 ,0.2mmolK 2 CO 3 and 1mLDMF, then reacted for 24 hours at 100°C, and the reaction mixture was simply separated to obtain 0.283g of 1,4-bis(4'-butylphenyl-1,3-butadiyne, yield 90%. Its NMR The data is: 1 HNMR (CDCl 3 ,400MHz)(δ,ppm): 0.92(t,J=7.6Hz,6H,CH 3 ),1.29-1.39(m,4H,CH 2 ),1.55-1.62(m,2H,CH 2 ), 2.61(t, J=7.6Hz, 4H, CH 2 ), 7.14(d, J=8Hz, 4H, ArH), 7.43(d, J=8Hz, 4H, ArH); 13 CNMR (CDCl 3 ,100MHz)(δ,ppm)144.4,132.4,128.5,118.9,81.5,73.4,35.5,33.3,22.3,13.9; low-resolution mass spectrum [LRMS(ESI+)] data: m / z=314.20 (the calculated value is 314.46 ).
Embodiment 3
[0071] Embodiment three: in reaction flask, add 2mmol4-ethylphenylacetylene, 0.1mmolCuCl 2 ,0.2mmolK 2 CO 3 and 1mLDMF, then reacted for 24 hours at 100°C, and the reaction mixture was simply separated to obtain 0.214g of 1,4-bis(4'-ethylphenyl-1,3-butadiyne, yield 83%. Its NMR The data is: 1 HNMR (CDCl 3 ,400MHz)(δ,ppm): 1.23(t,J=7.6Hz,6H,CH 3 ), 2.63-2.68 (t, J=7.6Hz, 4H, CH 2 ), 7.16(d, J=8Hz, 4H, ArH), 7.44(d, J=8Hz, 4H, ArH); 13 CNMR (CDCl 3 , 100 MHz) (δ, ppm) 145.7, 132.5, 128.0, 119.0, 81.5, 73.4, 28.9, 15.3; low resolution mass spectrum [LRMS (ESI+)] data: m / z = 258.14 (calculated value 258.36).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com