Hemoside compounds and application thereof
A technology of hualuoside and its compounds, which are applied in the field of hualuoside compounds and their applications, to achieve the effect of increasing curative effect, significantly multidrug-resistant tumor cells, and sensitizing anticancer drugs to inhibit multidrug-resistant tumor cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
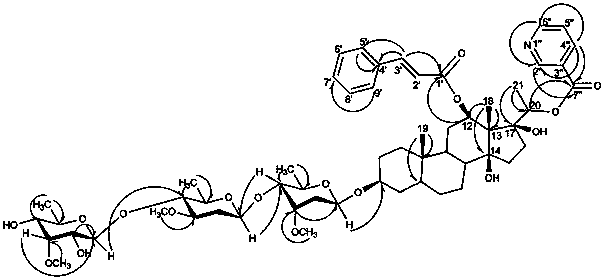
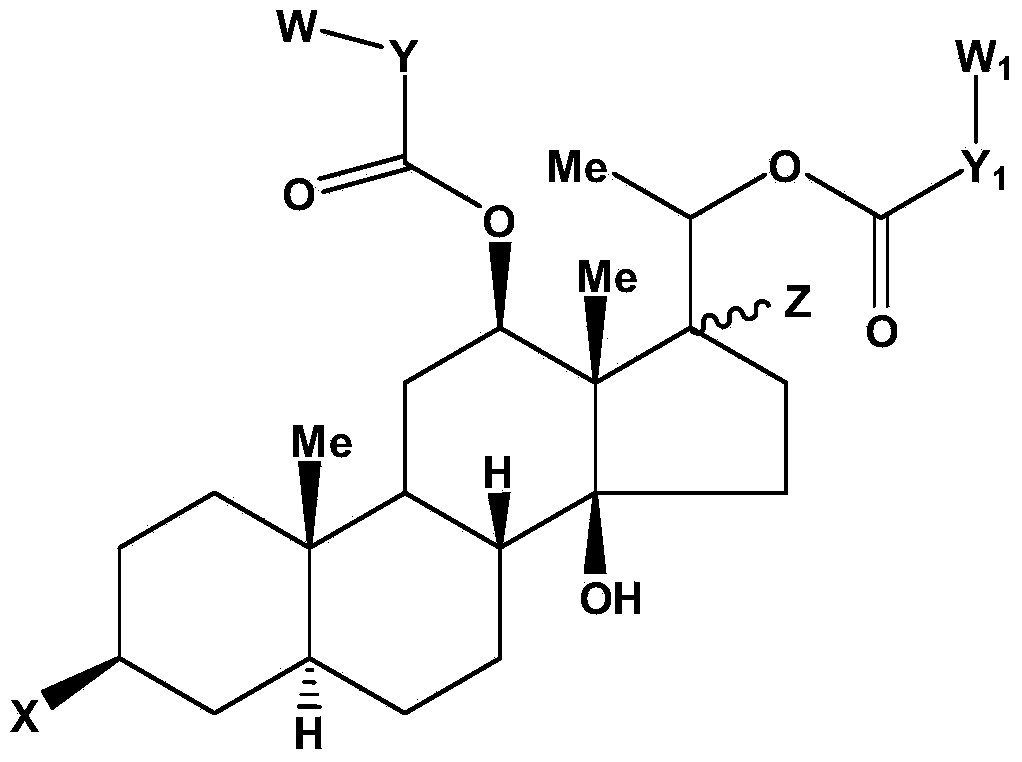
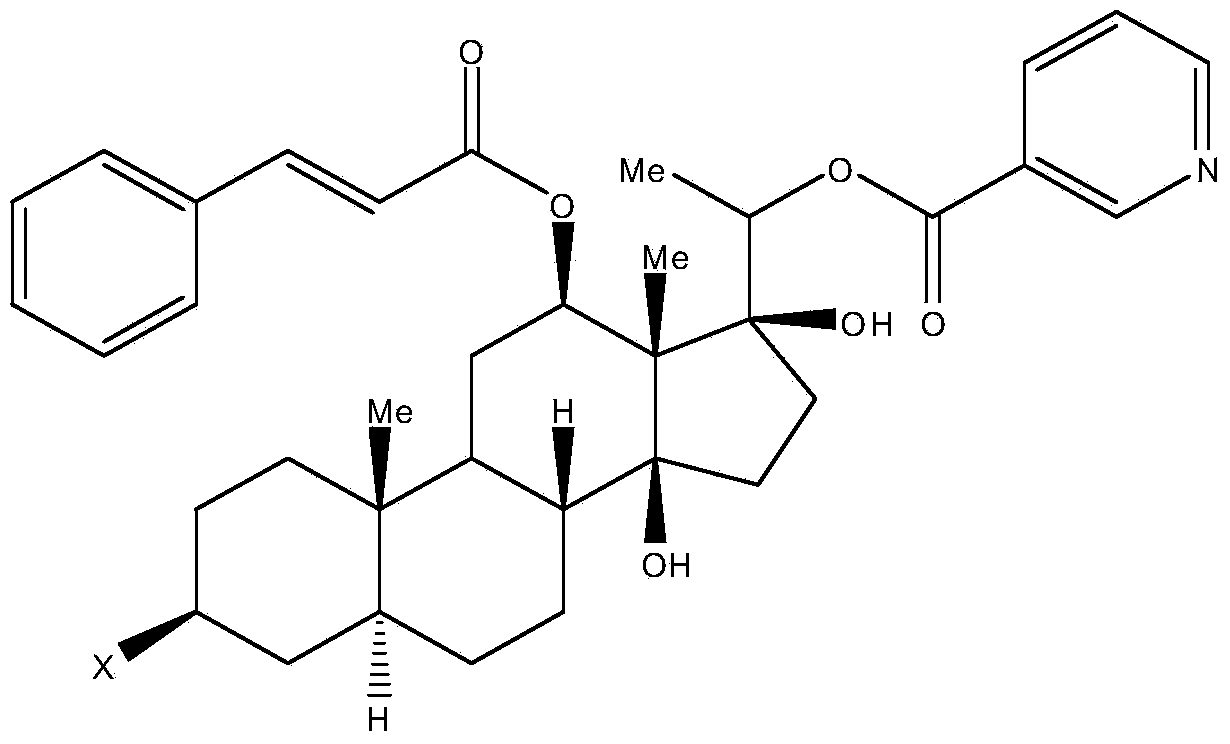
[0023] Hualuoside compound, the structural general formula of described Hualuoside compound is as follows:
[0024]
[0025] In the formula: thick solid line represents β substituent, dotted line represents α substituent, corrugated line represents α substituent or β substituent, Me is methyl; X is hydroxyl or sugar chain; Z is hydrogen or hydroxyl; Y , Y 1 are independently hydrocarbon groups with 0 to 4 carbons; W and W1 are independently aromatic hydrocarbon groups or heterocyclic aromatic hydrocarbon groups.
[0026] Preferably, Y, Y in the general formula of the above-mentioned hualuoside compounds 1 independently contain at most one ethylenic bond; in particular, Y is vinyl.
[0027] Preferably, W and W in the general formula of the above-mentioned hualuoside compounds 1 is independently a phenyl group or a heterocyclic aromatic hydrocarbon group in which one C atom is replaced by a N, O or S atom.
[0028] Preferably, the sugar chains in the general formula of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com