3,5-Difluorotyrosine translation system and its application
A technology of difluorotyrosine and translation system, which is applied to the analysis by nuclear magnetic resonance, the introduction of foreign genetic material and enzymes by using vectors, and can solve the problem of low tyrosine phosphorylation ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
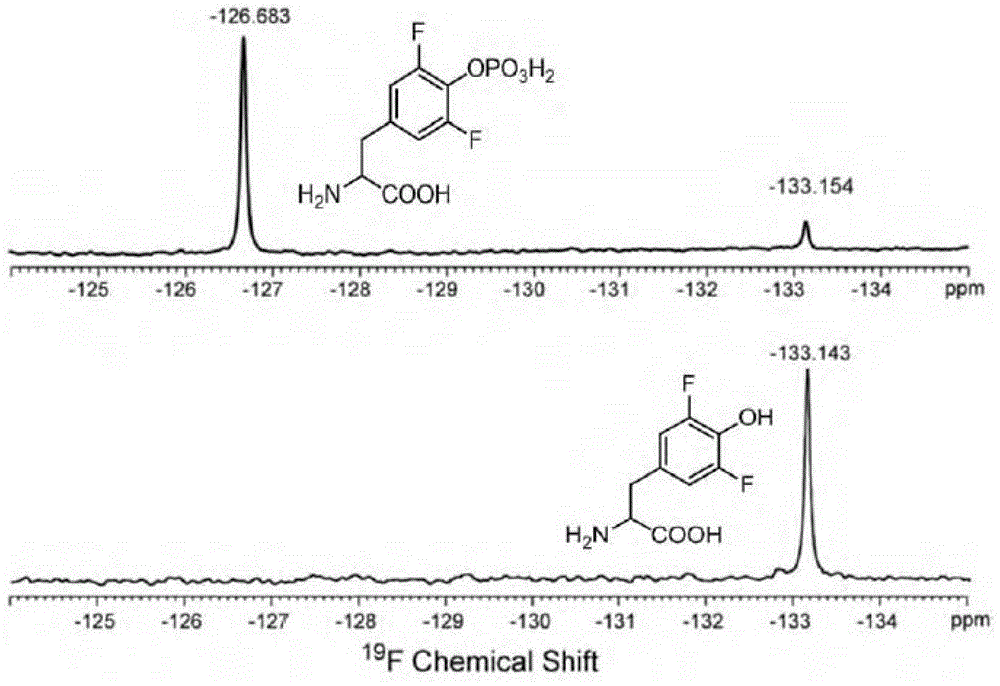
[0087] Embodiment 1: The chemical synthesis of o-phospho-3,5-difluorotyrosine (pF2Y)
[0088] Take 100mM phosphorus oxychloride, 100mM 3,5-difluorotyrosine (purchased from Shanghai Jier Biochemical Company) and 500mM sodium hydroxide, dissolve to 5mL, and then stir at room temperature for 1h. The aqueous phase was collected and separated and purified by HPLC to obtain a white powder with a yield of 10%. (YMC AA12S052503WT column, 12ml / min flow rate, from10%to90%CH3CN, 0.1%TFA(w / v) in water, over the course of 30 min).MS: m / z: 298[M+H]+; 1H-NMR (600 MHz, D2O): 7.03 (d, 2H) 4.01 (dd, 1H) 3.21 (m, 2H).
[0089] Unless otherwise specified, the chemical reagents required for the above synthesis reactions were purchased from Beijing Chemical Plant and were of analytical grade or above.
Embodiment 2
[0090] Example 2: Evolution of F2Y-specific aminoacyl-tRNA synthetases
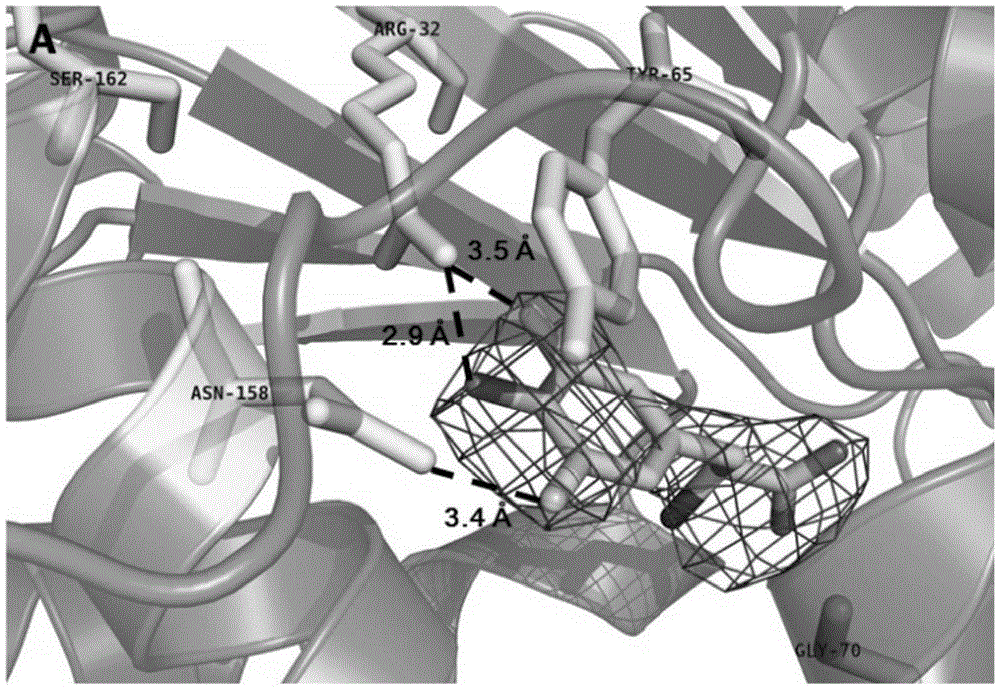
[0091] In order to site-specifically insert F2Y into the gene, it is necessary to introduce an aminoacyl-tRNA synthetase / tRNA orthogonal pair in the E.coli host cell used. Aminoacyl tRNA (MjtRNA CUA Y ) / tyrosyl tRNA synthetase (MjYRS, wild type, its amino acid sequence is SEQ ID NO: 2) pair. The MjYRS mutation library was constructed in the kanamycin-resistant pBK plasmid (purchased from the PeterG. Schultz laboratory of Scripps Research Institute, USA), and located between the promoter and terminator of E. coli glutamine synthetase on the plasmid. The synthetic enzyme mutant library used is the pBk-lib-jw1 library, and the construction method of the mutant library is: select 6 sites (Y32, Leu65, Phe108, Gln109, Asp158, and Leu162) on the MjYRS gene to introduce NNK mutation ( N=A+T+C+G; K=T+G), the other 6 sites (Ile63, Ala67, His70, Y114, Ile159, Vall64) were either randomly mutated to Gly or remaine...
Embodiment 3
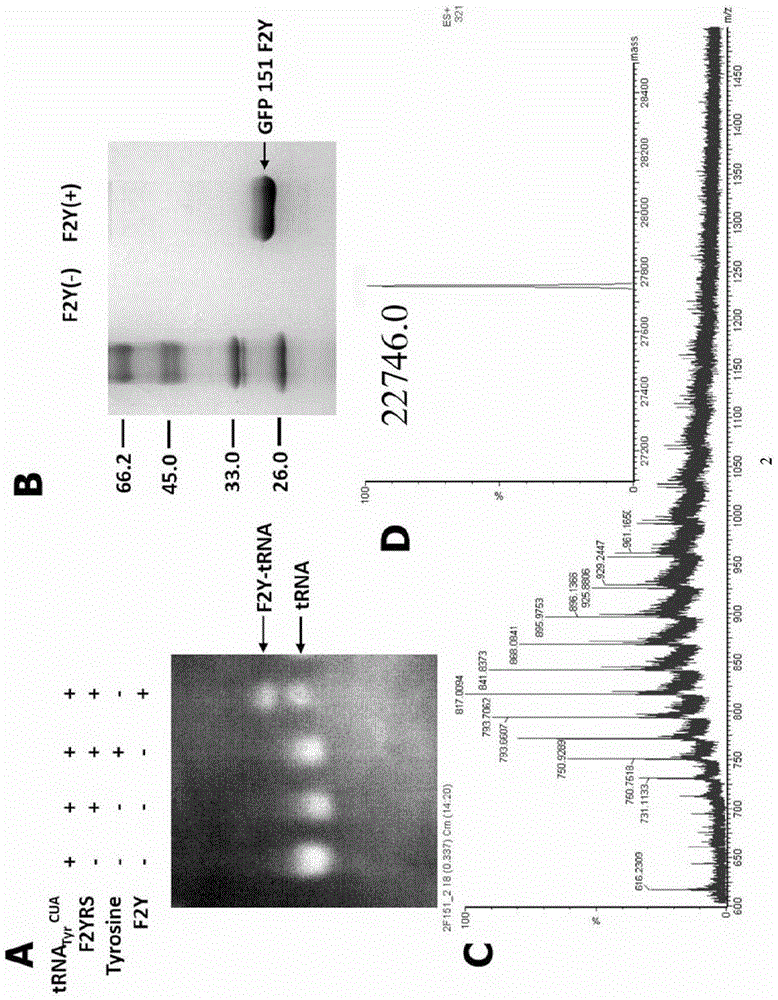
[0096] Example 3: Analysis of aminoacylation in vitro
[0097] In order to verify the high efficiency and fidelity of F2YRS integration of F2Y in the target protein, we performed an in vitro aminoacylation assay. Take 50mM sodium chloride, 20mM magnesium chloride, 4mM dithiothreitol, 2mM ATP, 10μM Tyr tRNA, 3μM F2YRS and 2mM tyrosine or F2Y, dissolve in 20mM Tris buffer, pH 8.0, and incubate at 37°C for 1h. The reaction solution was analyzed by 24h acid urea polyacrylamide gel electrophoresis, and the results were as follows: figure 2 As shown in A, F2YRS can only integrate F2Y, but not tyrosine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com