A kind of pharmaceutical composition and its application
A composition and drug technology, applied in the direction of drug combination, active ingredients of anhydride/acid/halide, nervous system diseases, etc., can solve the problems of child ADHD without atomoxetine hydrochloride and taurine, and achieve Effects of promoting SOD activity, reducing spontaneous hyperactivity behavior, and inhibiting MDA level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
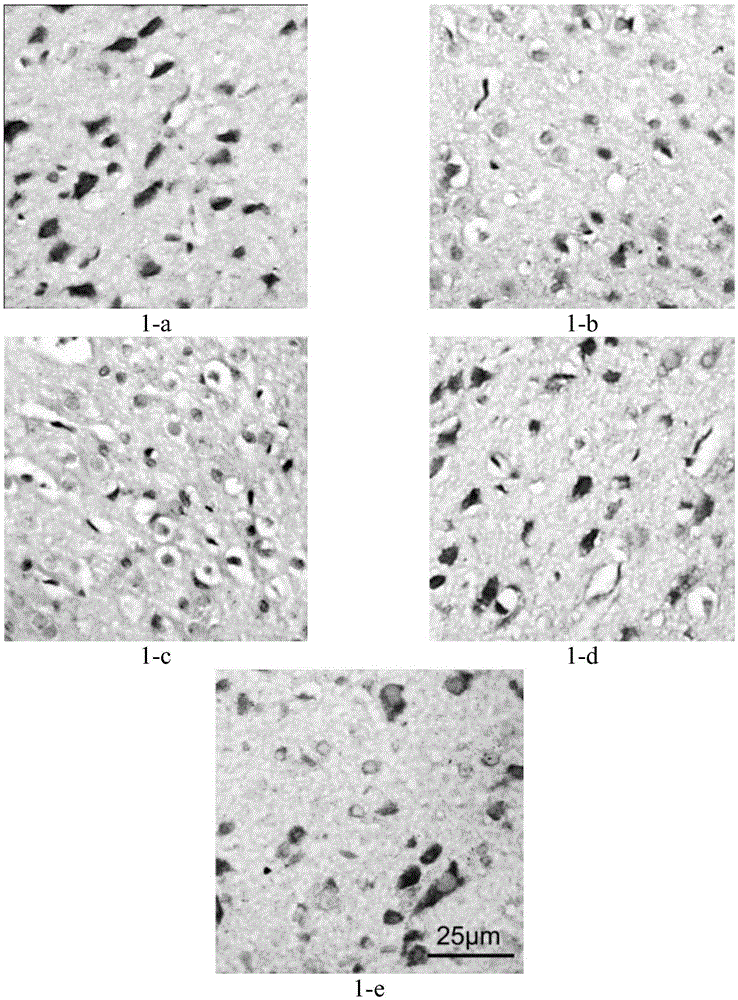
[0044] Embodiment 1 The effect of the pharmaceutical composition provided by the present invention on the juvenile stage SHR rat ADHD model
[0045] 1), experimental design
[0046] 54 newborn 21-day-old SHR rats ADHD model and 9 WKY rats were taken. After 3 days of adaptive feeding, 52 SHR rats were randomly divided into 6 groups according to body weight, 8-9 rats / group.
[0047] In the experiment, WKY rats were used as negative control;
[0048] The ADHD model of SHR rats without administration was used as the blank control;
[0049] The rats given only atomoxetine hydrochloride were used as the positive control group;
[0050] Three experimental groups with different dosages were set up: among them, experimental group 1 was given a high dose of the pharmaceutical composition, experimental group 2 was given a middle dose of the pharmaceutical composition, and experimental group 3 was given a low dose of the pharmaceutical composition. Administration dosage is as shown in ...
Embodiment 2
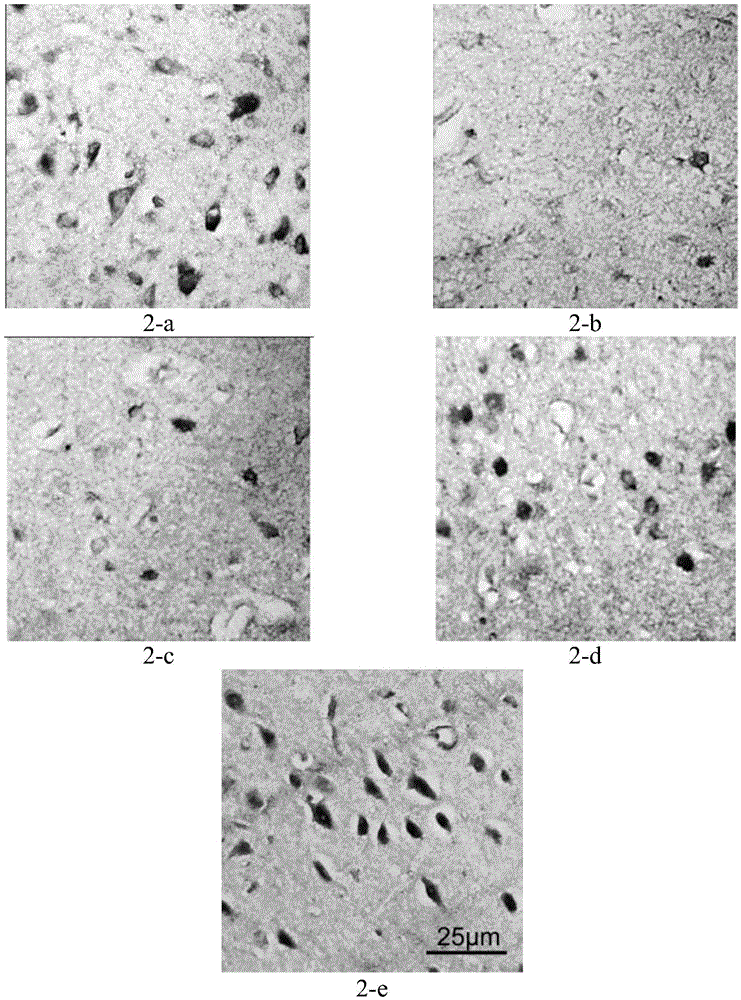
[0087] As shown in Table 5, compared with the blank control group, using atomoxetine hydrochloride alone can significantly (P<0.05) up-regulate the brain DBH, TH and Fos expression, and the pharmaceutical composition provided by the invention can be more significant (P<0.05) 0.01) up-regulate the expression of DBH, TH and Fos in the brain, suggesting that atomoxetine hydrochloride, taurine or taurine combined with atomoxetine have a positive effect on noradrenergic neurons and dopaminergic neurons in ADHD rats Protective effect, and the combination of the two drugs has a more significant effect than the single drug. Example 2 Effect of the pharmaceutical composition provided by the present invention on 6-OHDA neonatal rat ADHD model 1), animal modeling and group administration
[0088] When the newborn rats were 5 days old, they were randomly divided into 7 groups, which were: normal control group, model control group, positive control group, and experimental groups 1-3.
[0...
Embodiment 3
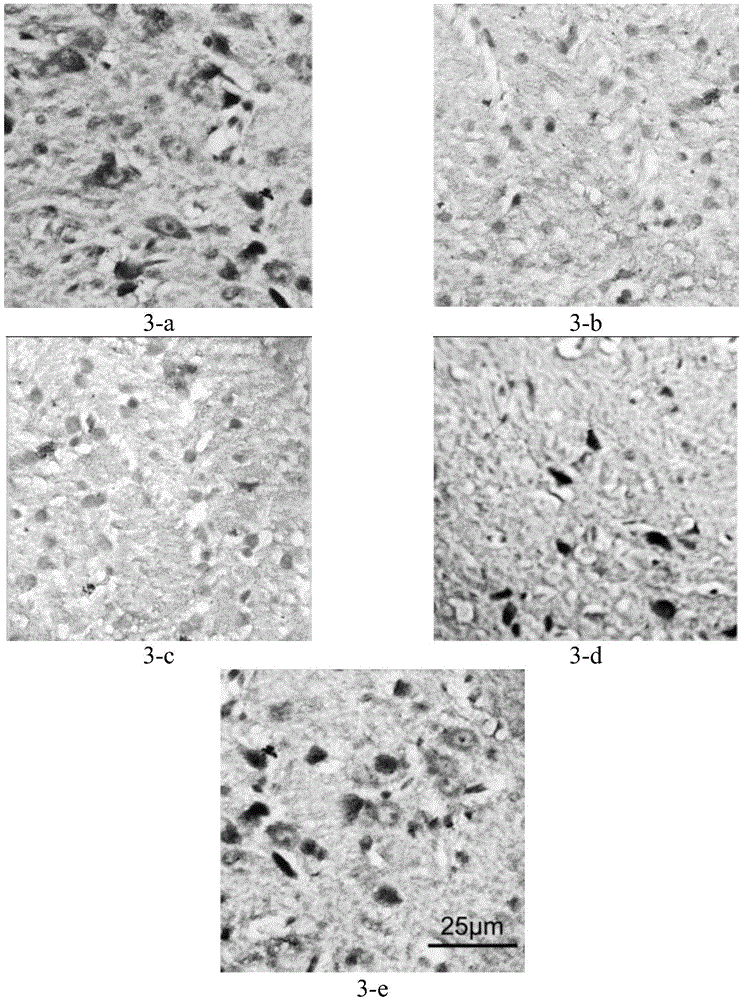
[0119] Embodiment 3 Pharmacokinetics and brain distribution research of the pharmaceutical composition provided by the present invention
[0120] In accordance with the relevant content requirements of the "Measures for the Administration of Drug Registration" and the "Guiding Principles for Non-clinical Pharmacokinetic Research of Chemical Drugs", SD rats were used as the subjects, and the effects of atomoxetine hydrochloride alone and in combination with taurine Preliminary study on pharmacokinetics and brain distribution.
[0121] Ten SD rats were randomly divided into two groups, A and B, and fasted for 12 hours before the experiment. Group A was orally administered atomoxetine hydrochloride at a dose of 5 mg / kg; group B was orally administered atomoxetine hydrochloride and taurine at a single dose of 5 mg / kg and 0.9 g / kg, respectively. During the administration process, ensure that the SD rats take the medicine completely. Collect blank blood before administration, collec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com