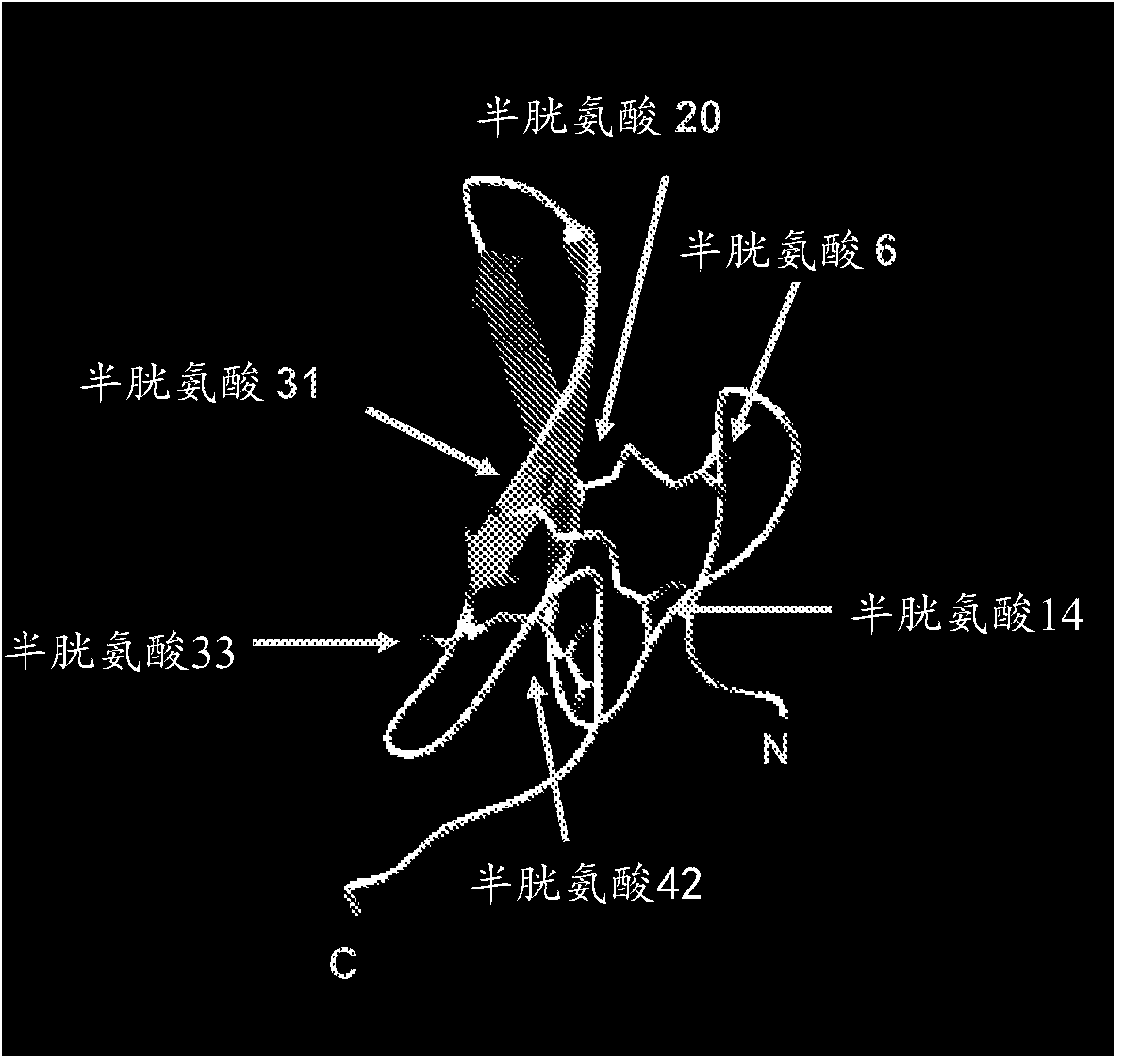
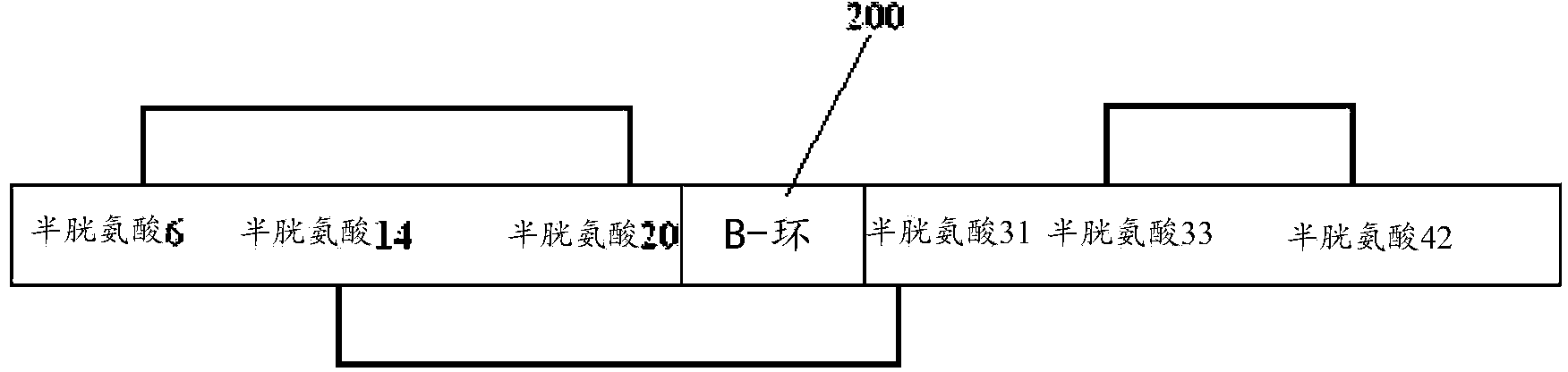
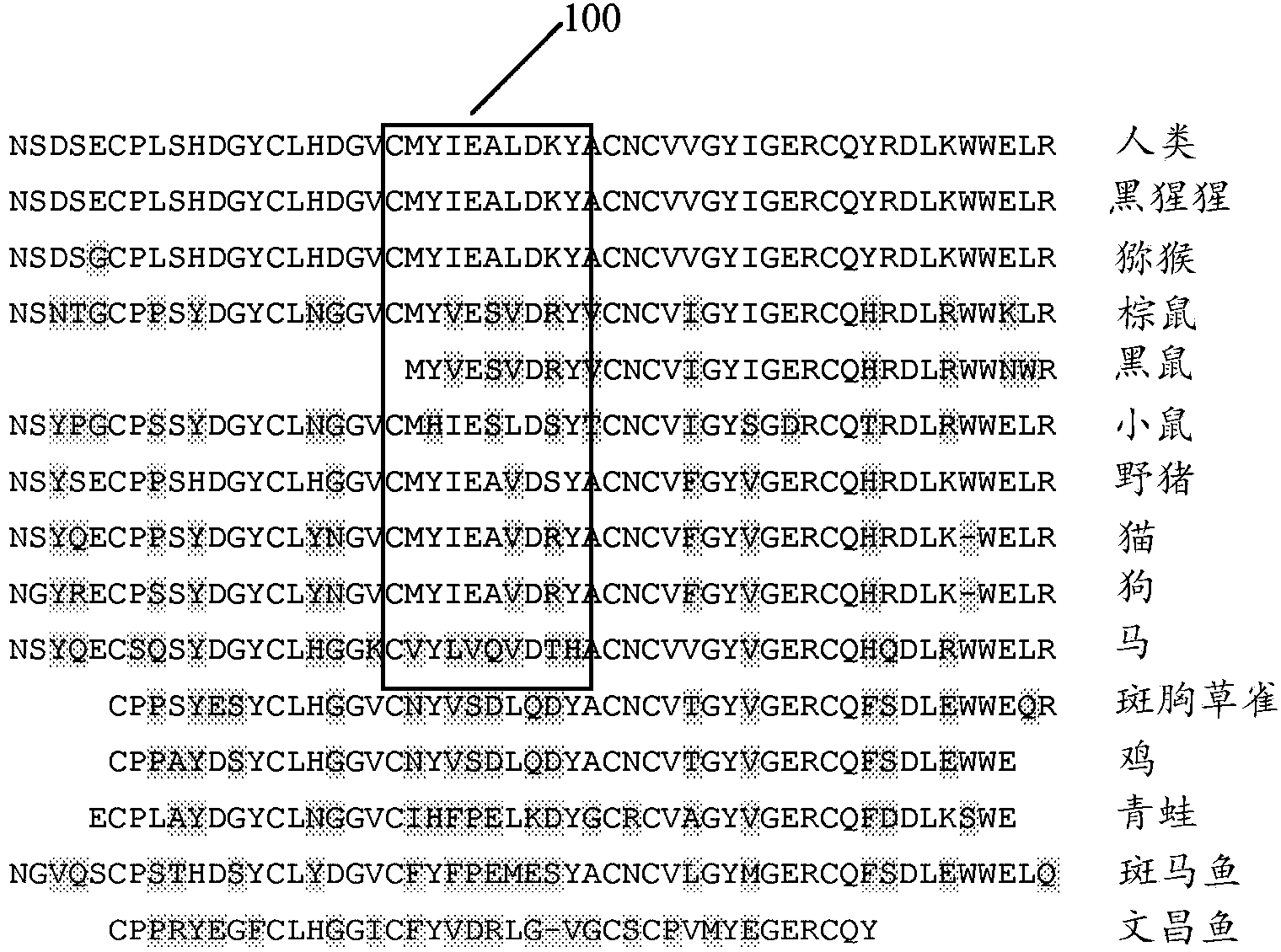Recombinant proteins and their therapeutic uses
A recombinant protein and protein technology, applied in the field of recombinant protein for the treatment of diseases, can solve problems such as no clinical effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
[0099] Example I: ELISA protocol
[0100] To determine whether a recombinant protein, such as the synthetic EGF-CT-B protein according to the present disclosure, is capable of displaying the B-loop of EGF in the correct conformation, two commercially available monoclonal antibodies (Santa Cruz Antibodies) were obtained , Cat. Nos. 10825 and 10827), two monoclonal antibodies known to block the binding of EGF to the EGF receptor. Without being bound by any particular theory, it is hypothesized that a number of sources of binding to the EGF receptor are in part via the region defined by residues Met21-Ala30.
[0101] In an exemplary embodiment, mAb 10825 and mAb 10827 at concentrations of 1 ug / ml and 2 ug / ml were used to bind recombinant EGF (rEGF) protein in an ELISA and optical density (OD) was measured at 450 nm. refer to Figure 6 The results are illustrated in a histogram. Such as Figure 6 It was shown that when rEGF was adsorbed on the ELISA plate, rEGF retained its na...
Embodiment II
[0105] Example II: Presentation of EGF Neutralizing Epitopes
[0106]To determine whether the recombinant protein EGF-CT-B vaccine expressing EGF at the end of the CT-B sequence interferes or affects any desired intrinsic properties of the EGF domain, in particular the presentation of the correct conformation of the Met21-Ala30 epitope of EGF , and whether it interferes or affects the ability of CT-B monomers to assemble into multimers (pentameric rings) under suitable physical-chemical conditions, and created 6 recombinant proteins, which are in CT-B The N-terminus (Test 1 to Test 3) or C-terminus (Test 4 to Test 6) of the sequence expressed the entire EGF coding region.
[0107] Test 1 and Test 4 contained the recombinant protein EGF-CT-B vaccine expressing the full-length EGF sequence directly on the CT-B domain. Test 2 and Test 5 contained a synthetic EGF-CT-B vaccine expressing the full-length EGF sequence separated from the CT-B domain by a short 3 amino acid peptide se...
Embodiment III
[0115] Example III: Multimer assembly of EGF-CT-B protein
[0116] To examine the effect of expressing domains including growth factors at the termini of CT-B-derived recombinant proteins on monomer-subunit assembly into multimers, synthetic proteins were made to test 1 under native conditions (non-reducing, not boiled). Run to test 6 on SDS-PAGE gel. Synthetic recombinant EGF-CT-B protein was then transferred to nitrocellulose membranes by electroblotting and probed with rabbit anti-CT-B antibody (as described in Example II). Binding of the HRP-labeled secondary anti-rabbit antibody was detected via light emission from the ECL substrate on the imaging membrane. Such as Figure 10 As shown, Western blotting confirmed the presence of high molecular weight CT-B, suggesting that the synthesized EGF-CT-B monomeric protein was able to assemble into multimers via the CT-B domain.
[0117] In separate experiments, replicate samples of native (non-boiled or non-reduced) CT-B protei...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com