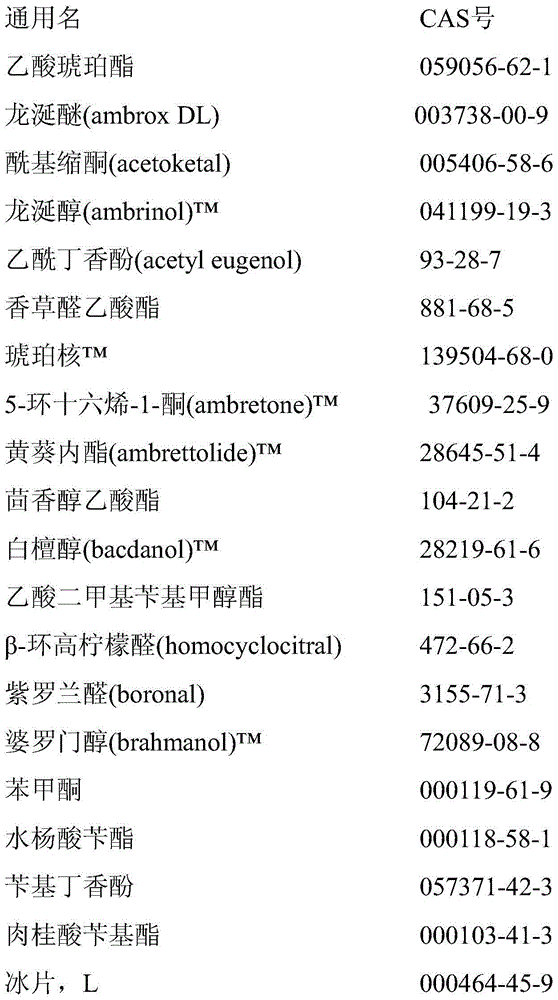
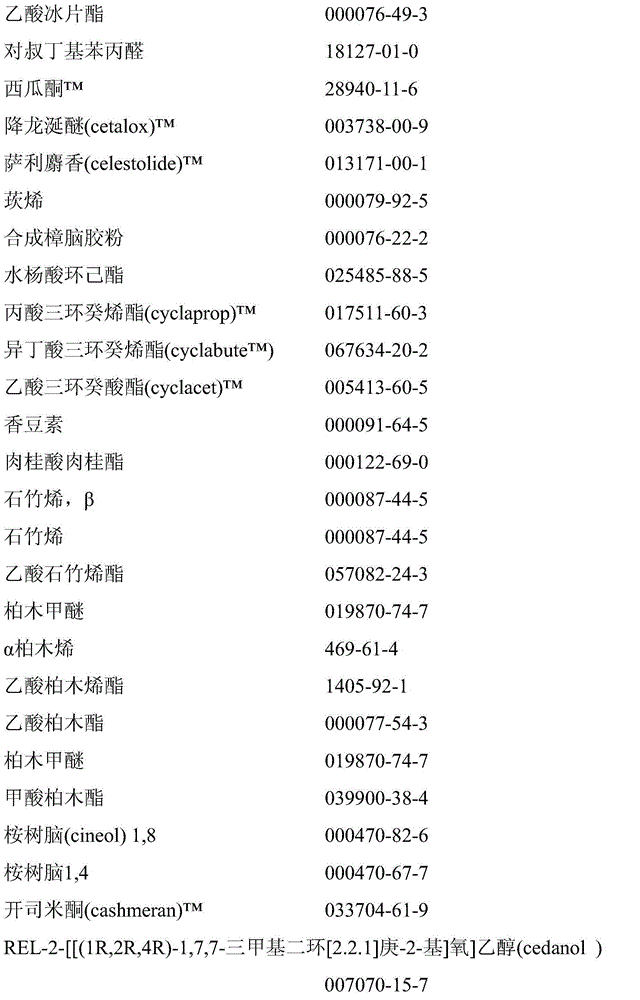
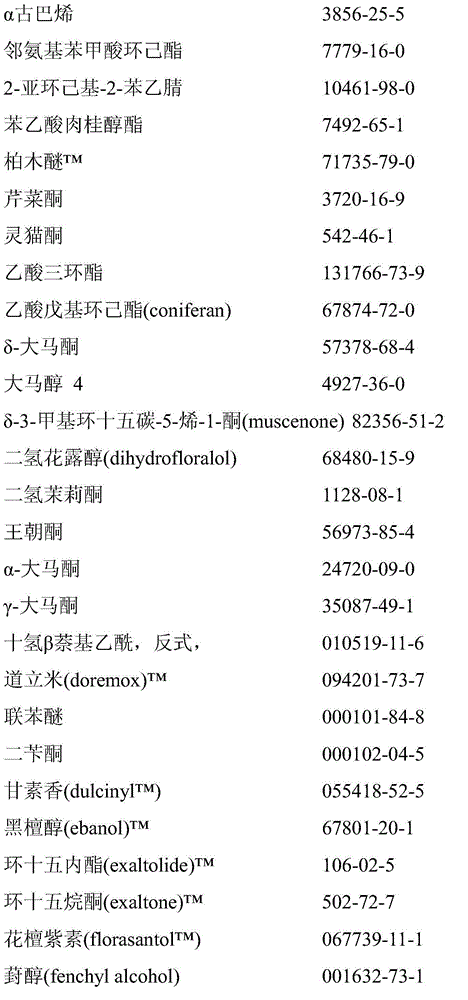
Microcapsules
A technology of microcapsules and monomers, applied in the direction of microcapsule preparation, microsphere preparation, coating detergent composition, etc., can solve problems such as unmentioned advantages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0362] Example 1: Preparation of capsules according to the invention (samples 1 to 3)
[0363] The aqueous phase was prepared by dissolving 4.0 g poly(vinyl alcohol) (hydrolyzed to 87-89%, Mw = 85000-124000 g / mol) in 196.0 g water. The oil phase was prepared by mixing 85.0 g of each perfume substance No. 1, 2 and 3, 13.7 g of 1,4-butanediol dimethacrylate, 13.1 g of methacrylic acid and 5.2 g of methyl methacrylate and 0.9 g prepared from lauroyl peroxide. The mixture was stirred until the lauroyl peroxide was completely dissolved.
[0364] The aqueous and oil phases were placed in a 500 mL-bath reactor equipped with a condenser, thermometer, nitrogen inlet and deflocculant plates (4 cm in diameter). During all processes, the mixture was stirred at 900 rpm and nitrogen was purged through the mixture to remove oxygen. First, the mixture was heated from room temperature to 35°C within 20 min and kept at 35°C for 1 hour. The resulting emulsion was then heated to 70°C within 3...
Embodiment 2
[0367] Example 2 (Comparative): Preparation of Capsules Using Crosslinker 1,4-Butanediol Diacrylate (Samples 4 and 5)
[0368] The aqueous phase was prepared by dissolving 4.0 g poly(vinyl alcohol) (hydrolyzed to 87-89%, Mw = 85000-124000 g / mol) in 196.0 g water. The oil phase was prepared by mixing 85.0 g of each fragrance substance No. 1 and 2, 13.7 g of 1,4-butanediol diacrylate, 13.1 g of methacrylic acid and 5.2 g of methyl methacrylate and 0.9 g of lauryl peroxide acyl to prepare. The mixture was stirred until the lauroyl peroxide was completely dissolved.
[0369] The aqueous and oil phases were placed in a 500 mL-bath reactor equipped with a condenser, thermometer, nitrogen inlet and deflocculant plates (4 cm in diameter). During all processes, the mixture was stirred at 900 rpm and nitrogen was purged through the mixture to remove oxygen. First, the mixture was heated from room temperature to 35 °C within 20 min and kept at 35 °C for 1 h. The resulting emulsion wa...
Embodiment 3
[0372] Embodiment 3 (comparison): Use the preparation of the capsule of cross-linking agent pentaerythritol triacrylate (sample 6)
[0373] The aqueous phase was prepared by dissolving 4.0 g poly(vinyl alcohol) (hydrolyzed to 87-89%, Mw = 85000-124000 g / mol) in 196.0 g water. The oil phase was prepared by mixing 85.0 g of each perfume substance No. 1, 2 or 3, 13.7 g of pentaerythritol triacrylate, 13.1 g of methacrylic acid and 5.2 g of methyl methacrylate and 0.9 g of lauroyl peroxide. The mixture was stirred until the lauroyl peroxide was completely dissolved.
[0374] The aqueous and oil phases were placed in a 500 mL-bath reactor equipped with a condenser, thermometer, nitrogen inlet and deflocculant plates (4 cm in diameter). During all processes, the mixture was stirred at 900 rpm and nitrogen was purged through the mixture to remove oxygen. First, the mixture was heated from room temperature to 35 °C within 20 min and kept at 35 °C for 1 h. The resulting emulsion was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com