Synthetic method for pyridine derivatives and application of derivatives
A pyridine and bonding technology, applied in electrical components, circuits, organic chemistry, etc., can solve the problems of concentration quenching triplet-triplet annihilation effect, reducing device luminous efficiency and brightness, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
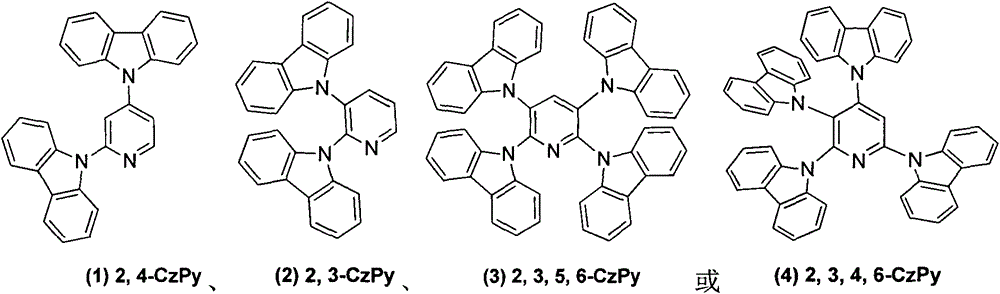
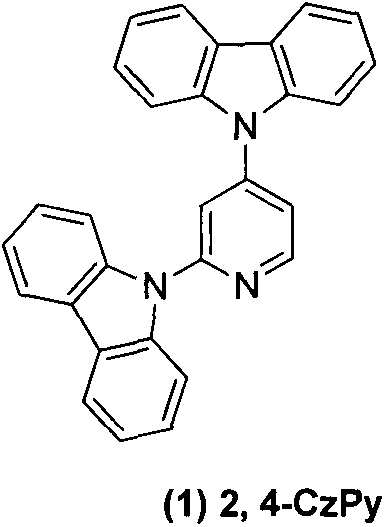
[0011] Embodiment 1: 2,4-dicarbazolylpyridine ( 1 )Synthesis
[0012] 2,4-Difluoropyridine (0.30g, 2.6mmol), potassium carbonate (2.2g, 15.6mmol), carbazole (0.96g, 5.7mmol), DMSO 10ml, heated to reflux at 150°C for 12h. Cooled to room temperature and poured into 200ml of water to precipitate a large amount of solid and stirred for 0.5h, suction filtered to obtain a white solid, purified by column chromatography to obtain 1.01g of white solid, yield 88%. 1 H NMR (CDCl 3 , 300MHz): δppm8.97 (d, 1H J = 5.4), 8.15 (t, 4H, J = 7.5Hz), 7.95 (d, 3H J = 7.5Hz), 7.68 (d, 3HJ = 8.1Hz), 7.49 (t, 4H J = 8.1 Hz), 7.36 (t, 4H J = 7.2 Hz).
[0013]
Embodiment 2
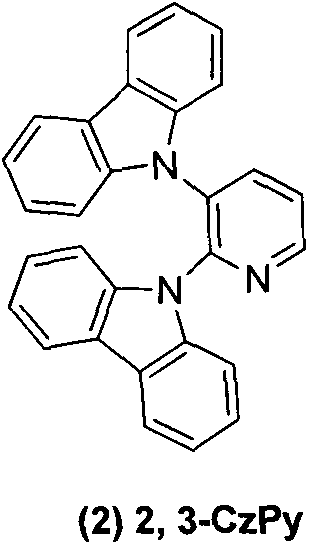
[0014] Embodiment 2: 2,3-dicarbazolylpyridine ( 2 )Synthesis
[0015] 2,3-Difluoropyridine (0.3g, 2.6mmol), the others were the same as in Example 1, and purified by column chromatography to obtain 0.91g of white solid with a yield of 85%. 1 H NMR (CDCl 3 , 300MHz): δppm8.87(d, 1HJ=3.6), 8.23(d, 1H, J=7.8Hz), 7.83-7.76(m, 4H), 7.66(t, 1HJ=7.5Hz), 7.27(t , 2H.J=6.6Hz), 7.09-6.99 (m, 10H).
[0016]
Embodiment 3
[0017] Embodiment 3: 2,3,5,6-tetracarbazolylpyridine ( 3 )Synthesis
[0018] 2,3,5,6-Tetrafluoropyridine (0.3g, 2.0mmol), the others were the same as Example 1, purified by column chromatography to obtain 1.3g of white solid, yield 90%. 1 H NMR (CDCl 3 , 300MHz): δppm8.76(s, 1H), 7.88-7.80(m, 8H), 7.52(d, 4H J=6.0), 7.27(t, 4HJ=3.3Hz), 7.14-7.10(m, 14H) , 7.09-7.00 (m, 4H).
[0019]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com