Triphenylethylene derivatives and application thereof
A technology for triphenylethylene and derivatives, which is applied in the field of triphenylethylene derivatives, can solve the problems of low performance of organic light-emitting devices and reduce the luminous efficiency of light-emitting layers, and achieves the effect of simplifying the structure and saving costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
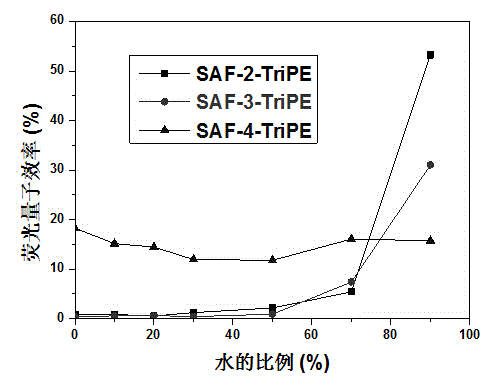
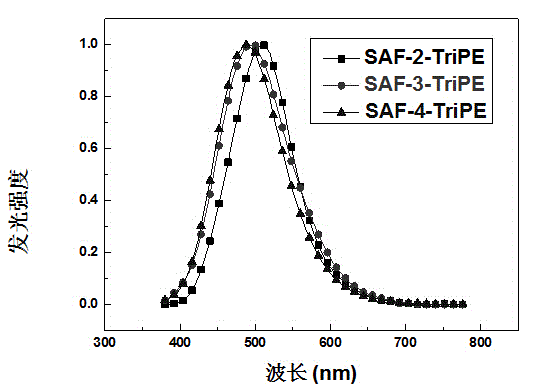
[0027] 2.0 g of ring-closed triphenylamine-2-boronate, 2.5 g of (2-bromo-1,1,2-phenyl)triphenylethylene, 3.0 g of tripotassium phosphate, 0.24 g of 2-bicyclohexylphosphine-2' , 6'-dimethoxybiphenyl (S-Phos), 0.1 g of tris(dibenzylideneacetone) dipalladium was dissolved in 120 ml of toluene and 12 ml of distilled water, refluxed under argon protection for 48 hours, cooled, washed with water , extracted with dichloromethane, the organic layer was dried with anhydrous sodium sulfate and spin-dried, passed through the column with a mixture of dichloromethane / petroleum ether, recrystallized with ethanol, and obtained 2.0 g of SAF-2-TriPE after sublimation.
Embodiment 2
[0029] 3.0 grams of ring-closed triphenylamine-3-boronate, 3.75 grams of (2-bromo-1,1,2-phenyl)triphenylethylene, 4.5 grams of tripotassium phosphate, 0.36 grams of S-Phos, 0.15 grams of tri( Dibenzylideneacetone) dipalladium was dissolved in 120 milliliters of toluene and 12 milliliters of distilled water, refluxed under argon protection for 48 hours, washed with water after cooling, extracted with dichloromethane, and the organic layer was dried with anhydrous sodium sulfate and spin-dried, and washed with dichloromethane Methyl chloride / petroleum ether mixture was passed through the column, recrystallized with ethanol, and 1.5 g of SAF-3-TriPE was obtained after sublimation.
Embodiment 3
[0031] 3.0 grams of ring-closed triphenylamine-4-boronate, 3.75 grams of (2-bromo-1,1,2-phenyl)triphenylethylene, 4.5 grams of tripotassium phosphate, 0.36 grams of S-Phos, 0.15 grams of tri( Dibenzylideneacetone) dipalladium was dissolved in 120 milliliters of toluene and 12 milliliters of distilled water, refluxed under argon protection for 48 hours, washed with water after cooling, extracted with dichloromethane, and the organic layer was dried with anhydrous sodium sulfate and spin-dried, and washed with dichloromethane The mixture of methyl chloride / petroleum ether was passed through the column, recrystallized with ethanol, and 1.0 g of SAF-4-TriPE was obtained after sublimation.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Maximum current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com