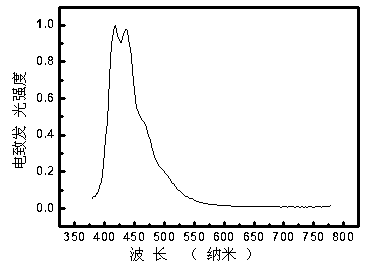
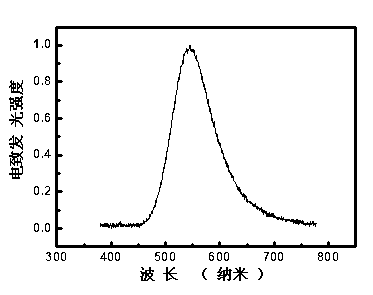
Hexafluorenylbenzene derivative and application thereof to organic photoelectric functional material
A technology of hexafluorenylbenzene and derivatives is applied in the field of organic optoelectronic functional materials and device preparation, which can solve the problems of low glass transition temperature and decreased stability, and achieve the effect of high-efficiency electroluminescence performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] Compound sample preparation
[0031] The hexafluorenylbenzene derivatives of the present invention can be prepared by Suzuki, Sonagashira coupling and other reactions to prepare substituted difluorene acetylenes, and then through dicobalt octacarbonyl (Co 2 (CO) 8 ) Catalyzed [2+2+2] cycloaddition reaction prepared. The raw materials used in this embodiment are known compounds, which can be purchased in the market, or can be synthesized by methods known in the art.
Embodiment 1
[0032] Example 1: Preparation of hexa-(9,9-di-n-hexylfluorene)benzene (2), the synthetic route is as follows
[0033]
[0034] (1) Preparation of hexa-(9,9-di-n-hexylfluorene)benzene
[0035] In the 100ml flask, add difluorene acetylene, weigh octacarbonyl dicobalt (Co 2 (CO) 8 ), add 80ml of anhydrous and oxygen-free 1,4-dioxane, protect with argon, stir and react at 120°C for 24 hours, pour into water after cooling and filter, the obtained solid is dissolved in chloroform, dried with petroleum ether : Chloroform=10:1 is the column separation of the eluent, and the resulting product is recrystallized with petroleum ether to obtain a white needle-like solid with a yield of 92%. Time-of-flight mass spectrometry: 2072.7. Elemental analysis: theoretical value C: 90.37%, H: 9.63%. Experimental value C: 90.05%, H: 9.44%.
Embodiment 2
[0036] Example 2: Preparation of hexa-(9,9-di-p-tolylfluorene)benzene (3), the synthetic route is as follows
[0037]
[0038] (1) Preparation of hexa-(9,9-di-p-tolylfluorene)benzene (3)
[0039] Referring to the preparation method of hexa-(9,9-n-hexylfluorene)benzene in Example 1, using petroleum ether: chloroform as the eluent = 4:1 (v / v) to pass through the column to obtain a white solid with a yield of 83%. Time-of-flight mass spectrum (m / z): 2144.0. Elemental analysis: theoretical value C: 94.08%, H: 5.92%, experimental value C: 93.86%, H: 5.81%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| luminance | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com