Synthesis method and application of pyridine and carboline derivative
A carboline and pyridine technology, applied in the application field of electrophosphorescence host in the field of electroluminescence, can solve the problems of reducing the luminous efficiency and brightness of the device, concentration quenching triplet state-triplet state annihilation effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
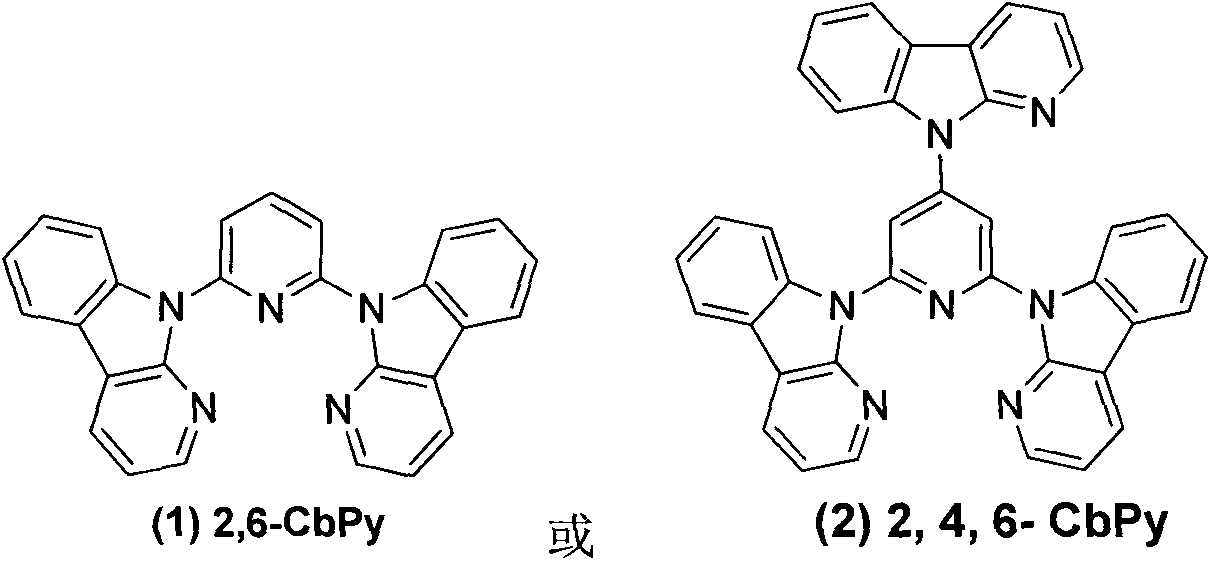
[0011] Embodiment 1: 2,6-dicarbolinopyridine ( 1 )Synthesis
[0012] 2,6-difluoropyridine (0.3g, 2.6mmol), potassium carbonate (2.2g, 15.6mmol), carboline (0.96g, 5.7mmol), DMSO 10ml, heated at reflux at 150°C for 12h. Cooled to room temperature and poured into 200ml of water to precipitate a large amount of solid, stirred for 0.5h, filtered with suction to obtain a white solid, purified by column chromatography to obtain 0.75g of white solid, yield 75%. 1 HNMR (CDCl 3 , 400MHz): δppm8.59(d, 2H, J=3.72Hz), 8.46-8.41(m, 4H), 8.32(d, 2H, J=7.76), 8.24-8.20(m, 1H), 8.10(d , 2H), 7.43-7.32(m, 6H).
[0013]
Embodiment 2
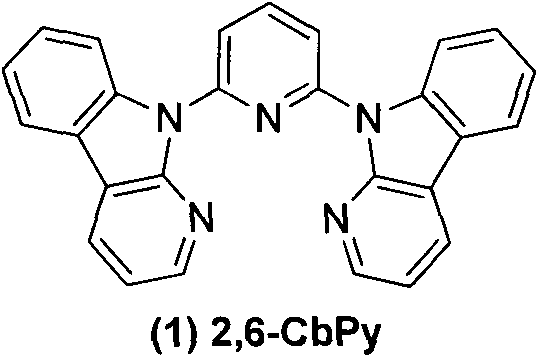
[0014] Embodiment 2: 2,4,6-tricarbolinopyridine ( 2 )Synthesis
[0015] 2,4,6-Trifluoropyridine (0.3g, 2.3mmol), the others were the same as Example 1, purified by column chromatography to obtain 0.81g of white solid, yield 61%. 1 H NMR (CDCl 3 , 400MHz): δppm8.95(s, 2H), 8.65(d, 2H, J=8.40), 8.59-8.57(m, 3H), 8.46-8.40(m, 4H), 8.18-8.11(m, 3H) , 7.64(t, 1H), 7.49-7.31(m, 8H).
[0016]
[0017] Table 1 is embodiment (1)-(2) The device data, the device structure is: MoO 3 (5nm) / NPB(60nm) / TCTA(5nm) / (1)-(2) : Firpic(13%-18%, 10nm) / TmPyPb(35nm) / CsCO 3 (2nm). The maximum current efficiency of the blue light device prepared as shown in Table 1 is as high as 42.6cd / A and the maximum energy efficiency is 37.12lm / W.
[0018]
[0019] Table 1
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com