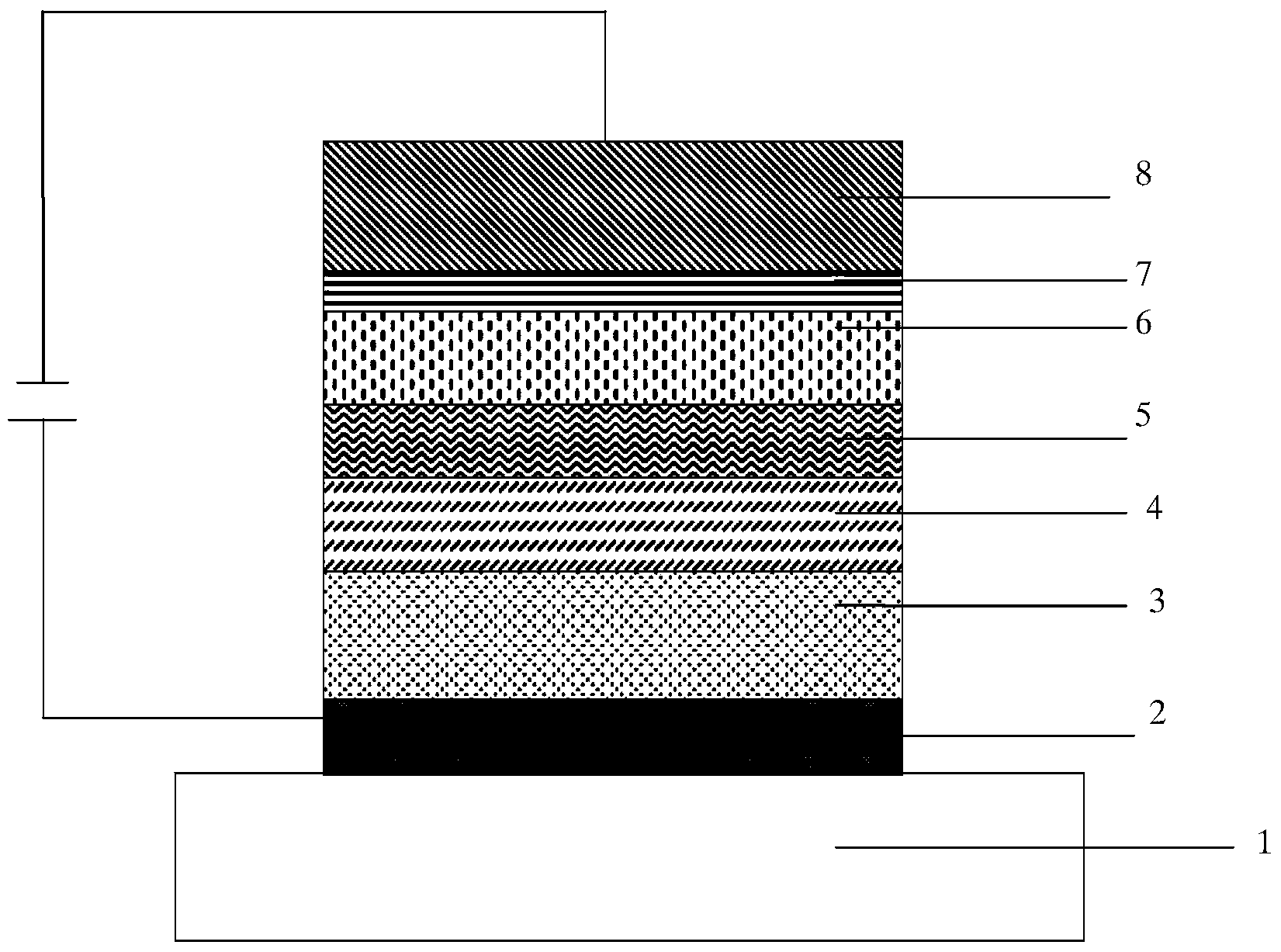
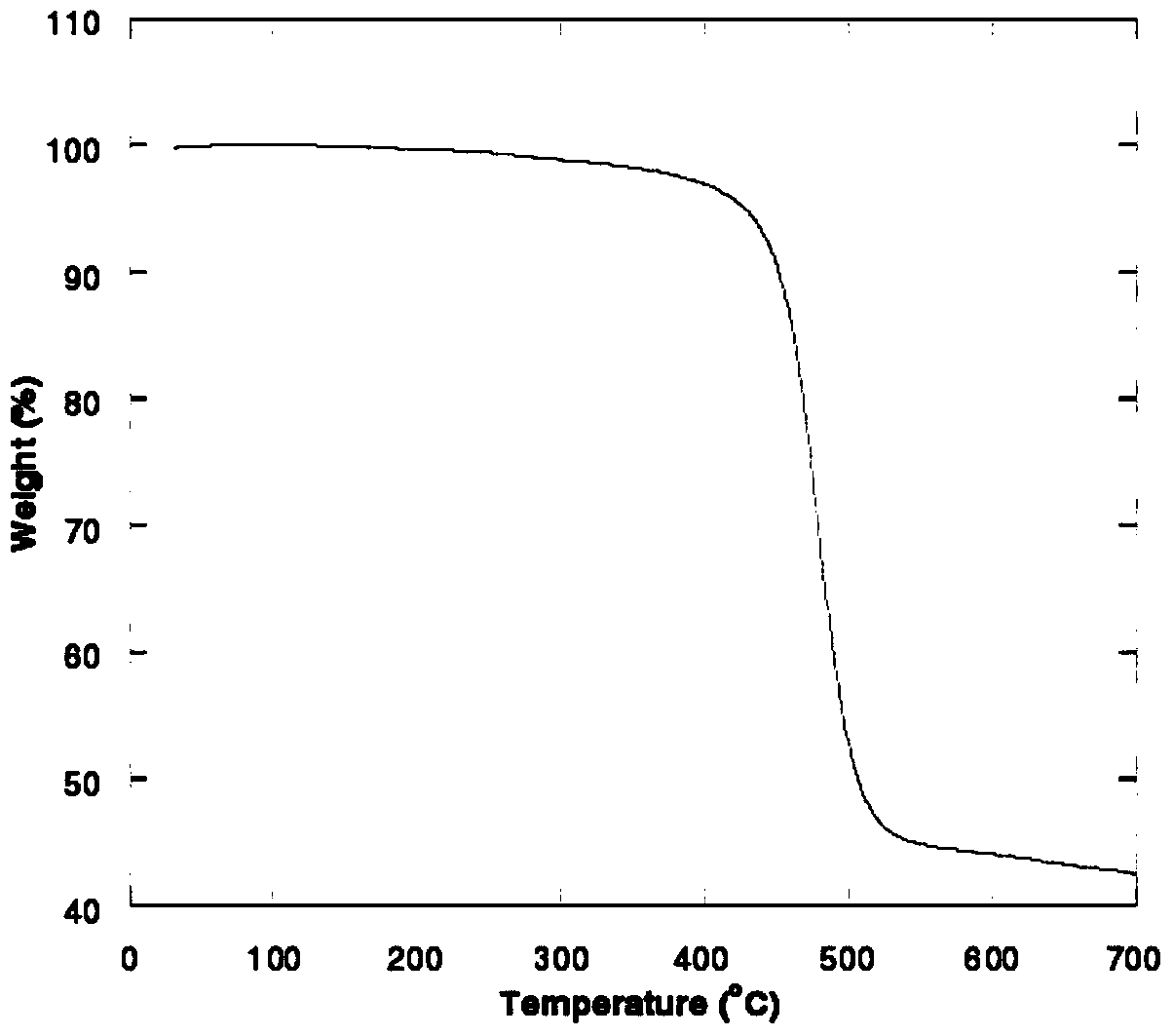
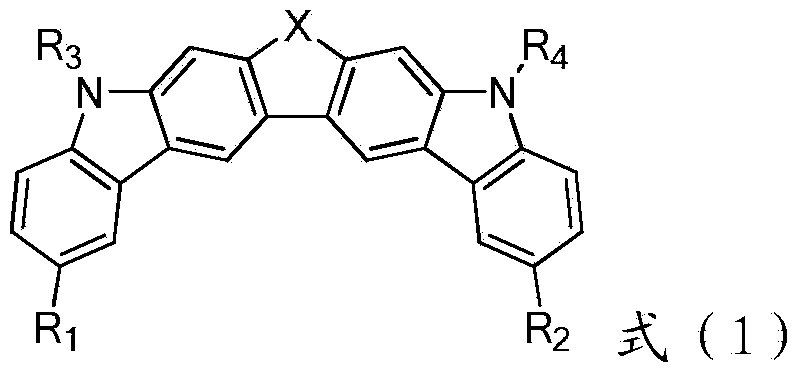
Oxidized thieno-bi-carbazole derivative and applications thereof
A technology of dicarbazole and derivatives, which is applied in the field of oxidative thienodicarbazole derivatives, can solve the problems of increasing the cost of device fabrication, achieve high glass transition temperature and decomposition temperature, good thermal stability, and improve luminous efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 15
[0058] Example 15, Synthesis of 9-diphenyl-5,9-dihydrothieno[2,3-b:5,4-b']dicarbazole-7,7-dioxide (Compound 1):
[0059] Take 5,9-dihydrothieno[2,3-b:5,4-b']dicarbazole-7,7-dioxide (19.7g, 50.0mmol) and sodium hydride (6.0g, 150mmol, 60% content), dissolved in 60mL N,N-dimethylformamide, and stirred under nitrogen gas for 1 hour to remove the oxygen in the reaction flask. Below 0°C, bromobenzene (23.0 g, 150 mmol) was added dropwise, and the reaction process was tracked and detected by TLC. After reacting for 3 hours, 150 mL of deionized water was slowly added to the reaction solution, and 26.3 g of off-white solid powder was obtained by filtration. The crude product was sublimated and purified in a chemical vapor deposition system at 320°C to obtain 24.6 g of off-white solid powder, with a yield of 90%. ). Using DEI-MS to identify the compound, formula C 36 h 22 N 2 o 2 S, detection value [M+1] + =546.96, the calculated value is 546.64.
Embodiment 25
[0060] Example 25,9-two (naphthalene-1-yl)-5,9-dihydrothieno[2,3-b:5,4-b']dicarbazole-7,7-dioxide (compound 2)
[0061] Use 5,9-dihydrothieno[2,3-b:5,4-b']dicarbazole-7,7-dioxide and 1-bromonaphthalene as starting materials according to compound 1 in Example 1 prepared by synthetic method. Using DEI-MS to identify the compound, formula C 44 h 26 N 2 o 2 S, detection value [M+1] + =647.33, the calculated value is 646.75.
Embodiment 35
[0062] Example 35, 9-bis(naphthalene-1-yl)-5,9-dihydrothieno[2,3-b:5,4-b']dicarbazole-7-oxide (compound 3)
[0063] Use 5,9-dihydrothieno[2,3-b:5,4-b'] dicarbazole-7-oxide and 1-bromonaphthalene as starting materials according to the synthetic method of compound 1 in Example 1 preparation. Using HR-MS to identify the compound, formula C 44 h 26 N 2 OS, detection value [M+1] + =631.29, the calculated value is 630.76.
PUM
| Property | Measurement | Unit |
|---|---|---|
| luminance | aaaaa | aaaaa |
| luminous efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com