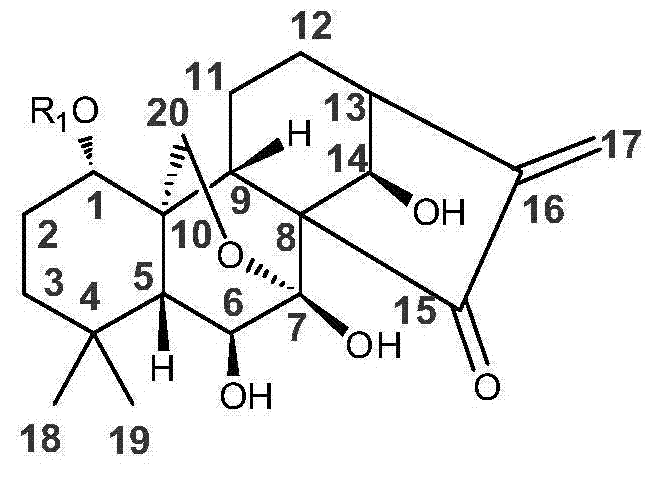
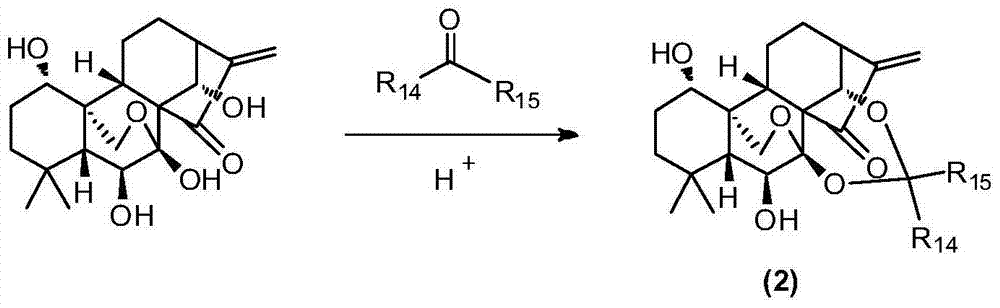
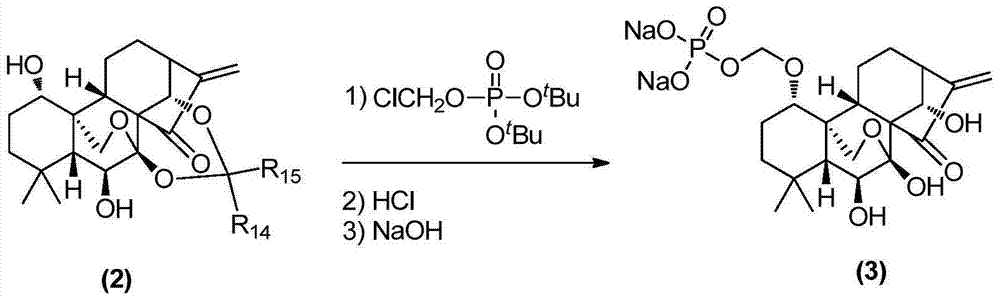
Water-soluble rubescensin a derivative and preparation method thereof
A technology of oridonin A and its derivatives, which is applied in the field of natural medicine and medicinal chemistry, and can solve the problems of oridonin A, such as difficulty, poor solubility, and limited application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1, the preparation of compound (3)
[0045] When R1 represents CH 2 PO 3 Na 2 , the preparation of oridonin A derivative (3) shown in general formula (1): under nitrogen protection, temperature control 0 ℃, 9mg NaH (0.370mmol) was added to 100mg propylidene-protected oridonin A (0.247mmol) and 5ml THF, stirred for 30min, then added 83mg O-di-tert-butyl chloromethyl phosphate (0.321mmol), after dropping, continued to stir for 1h, returned to room temperature, stirred for 10h, and evaporated to dryness. The residue is put on macroporous adsorption resin column AB-8, washed with water with a pH value of 4, then with water with a pH of 7.0, and finally eluted with ethanol, concentrated to give 105 mg of a white solid, which was dissolved in methanol and The strong acidic cationic resin was stirred for 24 hours, filtered, the filtrate was concentrated, purified by reverse phase, eluted with methanol / water, and neutralized with 1M NaOH to obtain 79 mg of Rubesce...
Embodiment 2
[0047] Embodiment 2, the preparation of compound (4) and its salt
[0048] When R1 stands for CONHCH 2 CH 2 CH 2 N(CH 3 ) 2 or CONHCH 2 CH 2 CH 2 N(CH 3 ) 2 ·HCl or CONHCH 2 CH 2 CH 2 N(CH 3 ) 2 When HOAc, the preparation of oridonin derivative (4) and its salt shown in general formula (1): under the protection of nitrogen, 136 μ l NMM (N-methylmorpholine) was added to 100 mg propylidene protected winter In a mixture of lobecidin (0.247mmol), 78mg N,N-dimethylpropylcarbamoylsuccinimide (0.321mmol) and 10ml acetonitrile, stir for 8h and evaporate to dryness. The residue is put on the macroporous adsorption resin column AB-8, washed with water with a pH value of 4 first, then washed with water with a pH of 7.0, and finally eluted with ethanol, concentrated to give 113 mg of white solid, which was dissolved in methanol and The strongly acidic cationic resin was stirred for 24 hours, filtered, the filtrate was concentrated, purified by reverse phase, and eluted with...
Embodiment 3
[0052] Embodiment 3, the preparation of compound (5) and its salt
[0053] When R1 represents CONHCH (CH 2 )(CH 2 CH 2 ) NCH 3 or CONHCH (CH 2 )(CH 2 CH 2 ) NCH 3 · HCl or CONHCH (CH 2 )(CH 2 CH 2 ) NCH 3 When HOAc, the preparation of oridonine A derivative (5) and its salt shown in general formula (1): under the protection of nitrogen, 136 μ l NMM (N-methylmorpholine) was added to 100 mg propylidene protected winter In a mixture of lobecidin (0.247mmol), 77mg of N-methylpyrrole-3-carbamoylsuccinimide (0.321mmol) and 10ml of acetonitrile, stir for 8h and evaporate to dryness. The residue is put on macroporous adsorption resin column AB-8, washed with water with a pH value of 4, then with water with a pH of 7.0, and finally eluted with ethanol, concentrated to give 109 mg of white solid, which was dissolved in methanol and Strongly acidic cationic resin was stirred for 24 hours, filtered, the filtrate was concentrated, purified by reverse phase, and eluted with meth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com