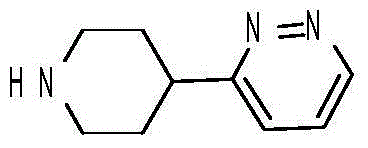
Preparation method for 3-(piperidine-4-yl)-pyridazine
A kind of technology of piperidine and pyridazine, applied in the field of preparation of pyridazine derivative 3--pyridazine, can solve problems such as synthesis difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
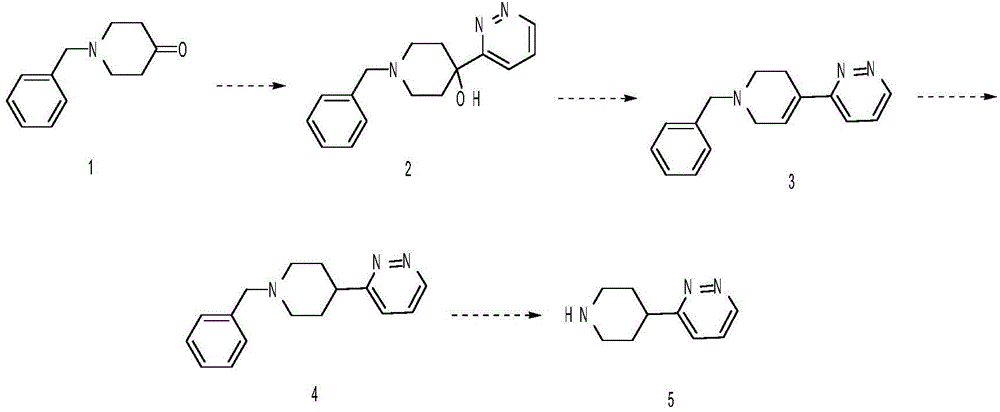
Embodiment 1
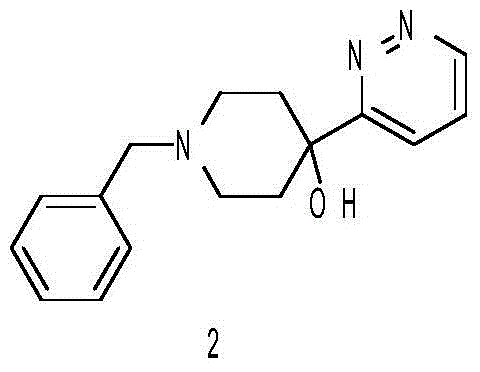
[0022] (1) Synthesis of 1-benzyl-4-(pyridazin-3-yl)piperidin-4-ol
[0023] Add 16g of 3-bromopyridazine into 190ml of anhydrous tetrahydrofuran, cool down to -78°C, add 50ml of 2.5M n-butyllithium dropwise, stir for 30min, then add 22g of N-benzylpiperidone solution in tetrahydrofuran dropwise, Stir at -78°C for 2 hours, warm up to room temperature, add saturated ammonium chloride, add water and ethyl acetate for extraction and separation, collect the organic phase, dry, concentrate, and the residue is put on a silica gel column to obtain 21g 1-benzyl-4-( Pyridazin-3-yl)piperidin-4-ol.
[0024] (2) Synthesis of 3-(1-benzyl-4-yl-1,2,3,6-tetrahydro)pyridazine
[0025] Add 20g of 1-benzyl-4-(pyridazin-3-yl)piperidin-4-ol to 200ml of hydrochloric acid, heat to reflux and stir for 10 hours, add aqueous sodium hydroxide solution to adjust pH=10, add ethyl acetate to extract The liquid was separated, the organic phase was collected, dried and concentrated to obtain 15 g of 3-(1-ben...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com