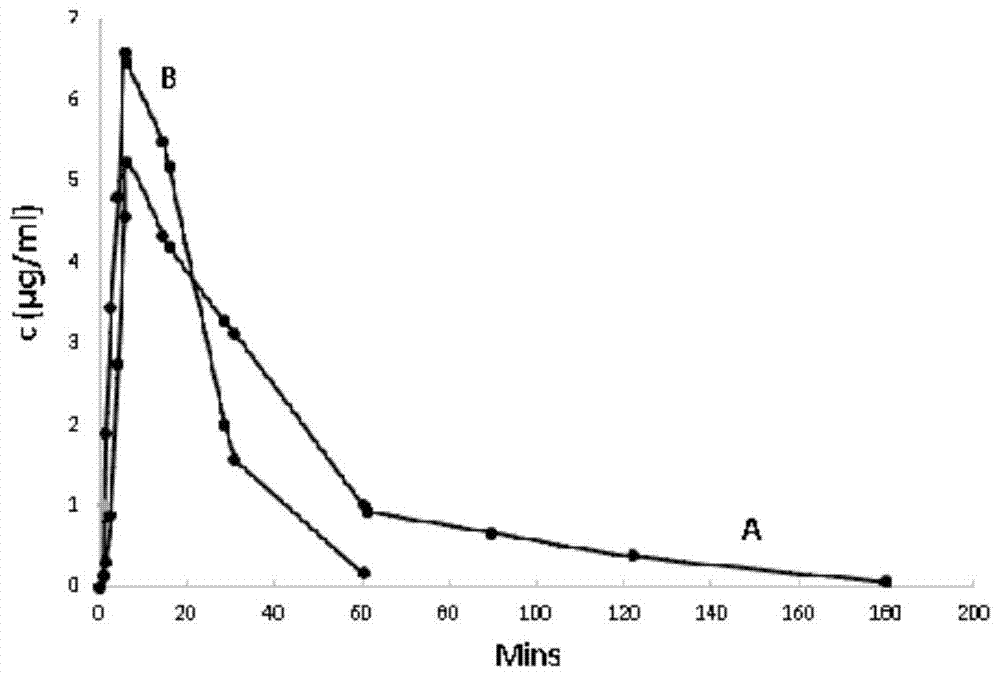
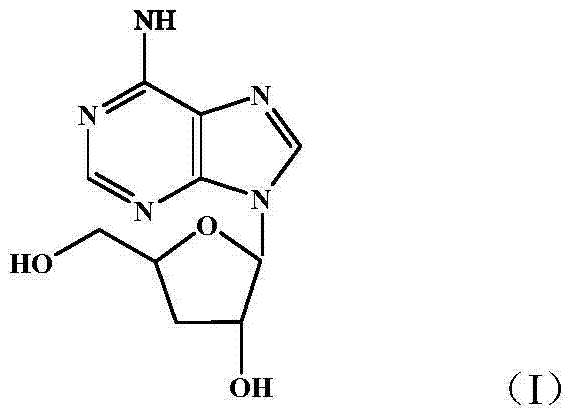
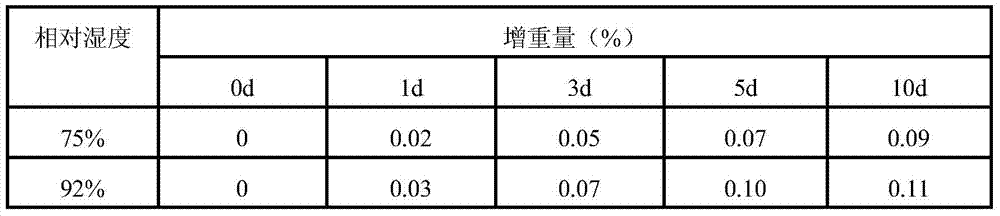
A kind of preparation method of cordycepin proliposome
A proliposome and cordycepin technology, applied in the field of medicine, can solve the problem of limiting pharmacological activity such as anti-tumor, achieve the effect of increasing tumor targeting performance, increasing activity, and avoiding degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1 Cordycepin liposome process screening
[0026] 1. Ethanol injection method: mix and dissolve lecithin and cholesterol in a mass ratio of 4:1 in an appropriate amount of absolute ethanol to obtain a lipid solution. Add an appropriate amount of cordycepin pH 7.4 phosphate buffer into the three-necked flask. Under stirring, slowly inject the above-mentioned lipid solution into the cordycepin buffer solution, continue to stir, and after heating up to volatilize ethanol, cordycepin liposomes are obtained with an encapsulation efficiency of 28.5%.
[0027] 2. Film dispersion method: dissolve lecithin and cholesterol in an appropriate amount of chloroform at a mass ratio of 4:1, remove the organic solvent with a rotary evaporator, and form a uniform phospholipid film in an eggplant-shaped bottle. Add cordycepin phosphate buffer, stir and elute the phospholipid membrane to obtain liposomes. The encapsulation efficiency was 33.2%.
[0028] 3. pH gradient method: ...
Embodiment 2
[0033] Example 2 Process optimization for preparation of cordycepin liposomes by reverse-phase evaporation combined with freeze-drying
[0034] (1) General method:
[0035] Lecithin and cholesterol are dissolved in an appropriate amount of chloroform (oil phase) according to a certain mass ratio, and cordycepin phosphate buffer (water phase) is added. Control the volume ratio of the oil phase to the water phase, short-time ultrasound for 5 minutes to form an emulsion, and then remove the organic solvent on a rotary evaporator under reduced pressure to form a colloidal state, add an appropriate amount of distilled water, and continue to rotate to hydrate to form liposomes. Add an appropriate amount of lyoprotectant to the above-mentioned cordycepin liposome suspension, pre-freeze at -40°C for 6-12 hours, vacuumize, and continue to freeze-dry at -40°C for 40 hours to obtain cordycepin proliposomes.
[0036] (2) Optimization of liposome preparation process:
[0037] In order to...
Embodiment 3
[0069] Embodiment 3 The preparation method of cordycepin proliposome
[0070] (1) Preparation method
[0071] Dissolve 196.8 mg of lecithin and 32 mg of cholesterol in 14.0 ml of chloroform as the oil phase; 2.5 ml of cordycepin phosphate buffer with a concentration of 0.5 mg / mL as the water phase; mix the oil phase with the water phase, and ultrasonicate for 5 minutes to form milk. Then, under the condition of 37°C, remove chloroform under reduced pressure on a rotary evaporator with a vacuum degree of 0.05-0.06Mpa and a rotation speed of 60rpm, and form a colloidal state on the bottle wall without the pungent smell of chloroform. Add 15ml of distilled water and continue to rotate. Evaporate to form cordycepin liposome suspension;
[0072] Add 2ml of 8% lactose solution to the above-mentioned cordycepin liposome suspension, pre-freeze at -40°C for 6h, vacuumize, and continue to freeze-dry at -40°C for 40h to obtain cordycepin proliposomes.
[0073] (2) Physical and chemical...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com