A kind of Ipagliflozin tablet and preparation method thereof
A technology of ixagliflozin and prescription, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc. It can solve the problems of low dissolution rate, unstable quality, and particle size requirements, etc. problems, to achieve simple production process, high drug dissolution rate, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
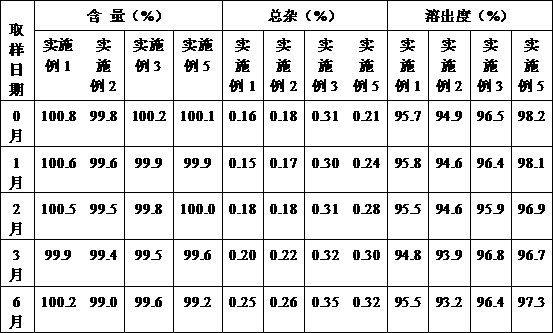
Examples
Embodiment 1
[0031] Ipagliflozin Tablet Core Prescription (Specification: 50mg):
[0032] Ipagliflozin 50.0 g
[0033] Polyethylene glycol 6000 100.0 g
[0034] Crospovidone 16.0 g
[0035] Magnesium stearate 2.0 g
[0036] A total of 1000 pieces were made
[0037] Coating Solution Prescription:
[0038] Opadry 85F18422 6.0 g
[0039] Purified water 44.0 g
[0040] Preparation:
[0041] 1. Preparation of drug-containing tablet cores
[0042] ① Processing of raw and auxiliary materials: Mix Ipagliflozin and polyethylene glycol 6000 evenly, and sieve the cross-linked povidone with an 80-mesh sieve for later use;
[0043] ② Add 1.2kg of absolute ethanol to the above mixture of Ipagliflozin and polyethylene glycol 6000, heat in a water bath at 65°C, and stir until completely dissolved;
[0044] ③ After complete dissolution, raise the temperature to 90°C to recover absolute ethanol. When the absolute ethanol is recovered to 60-80ml, add 12.0g crospovidone and mix well to make a soft m...
Embodiment 2
[0054] Ipagliflozin Tablet Core Prescription (Specification: 100mg):
[0055] Ipagliflozin 100.0 g (dry and pure)
[0056] Macrogol 4000 100.0 g
[0057] Croscarmellose Sodium 20.0 g
[0058] Micronized silica gel 2.0 g
[0059] A total of 1000 pieces were made
[0060] Coating Solution Prescription:
[0061] Opadry 85F42129 14.0 g
[0062] 75% ethanol 186.0 g
[0063] Preparation:
[0064] 1. Preparation of drug-containing tablet cores
[0065] ① Processing of raw and auxiliary materials: Mix Ipagliflozin hemihydrate and polyethylene glycol 4000 evenly, and sieve croscarmellose sodium with an 80-mesh sieve for later use;
[0066] ②Add 1.5kg of absolute ethanol to the above mixture of Ipagliflozin Hemihydrate and Polyethylene Glycol 4000, heat in a water bath at 60°C, and stir until completely dissolved;
[0067] ③ After complete dissolution, raise the temperature to 88°C to recover absolute ethanol, and when the absolute ethanol is recovered to 85-100ml, add 15.0g ...
Embodiment 3
[0077] Ipagliflozin Tablet Core Prescription (Specification: 100mg):
[0078] Ipagliflozin 100.0 g
[0079] Povidone 150.0 g
[0080] Croscarmellose Sodium 30.0 g
[0081] Magnesium stearate 3.0 g
[0082] A total of 1000 pieces were made
[0083] Coating Solution Prescription:
[0084] Opadry 85F18422 20.0 g
[0085] 70% ethanol 230.0 g
[0086] Preparation:
[0087] 1. Preparation of drug-containing tablet cores
[0088] ① Processing of raw and auxiliary materials: mix iggliflozin and povidone evenly, and sieve croscarmellose sodium with a 60-mesh sieve for later use;
[0089] ② Add 3.0kg of absolute ethanol to the above mixture of ixagliflozin and povidone, heat in a water bath at 75°C, and stir until completely dissolved;
[0090] ③ After complete dissolution, heat up to 90°C to recover absolute ethanol. When the absolute ethanol is recovered to 120-180ml, add 20.0g of cross-linked carmellose sodium and mix well to make a soft material;
[0091] ④ Wet granulati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com