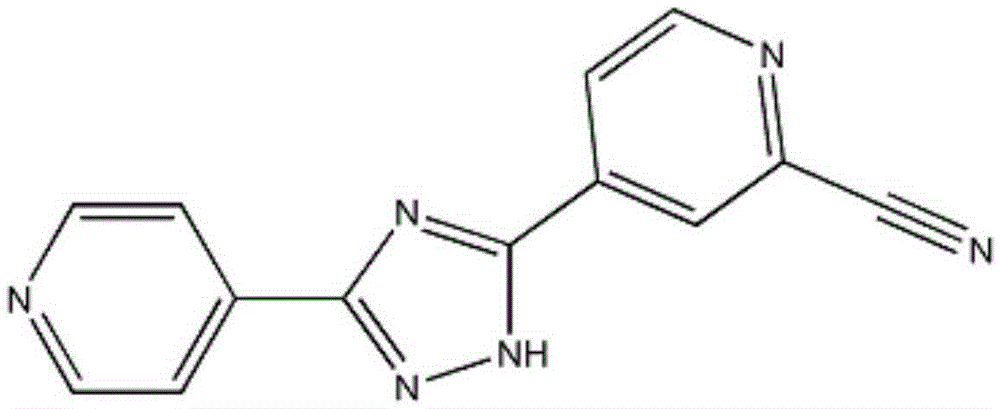
Topiroxostat dispersible tablets and preparing method thereof
A technology of topinostat and dispersible tablets, which is applied in the field of topinostat dispersible tablets and its preparation, can solve the problems of difficult treatment for patients, influence on drug absorption, slow disintegration speed, etc., and achieve good drug safety and human body absorption Good, fast disintegration effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
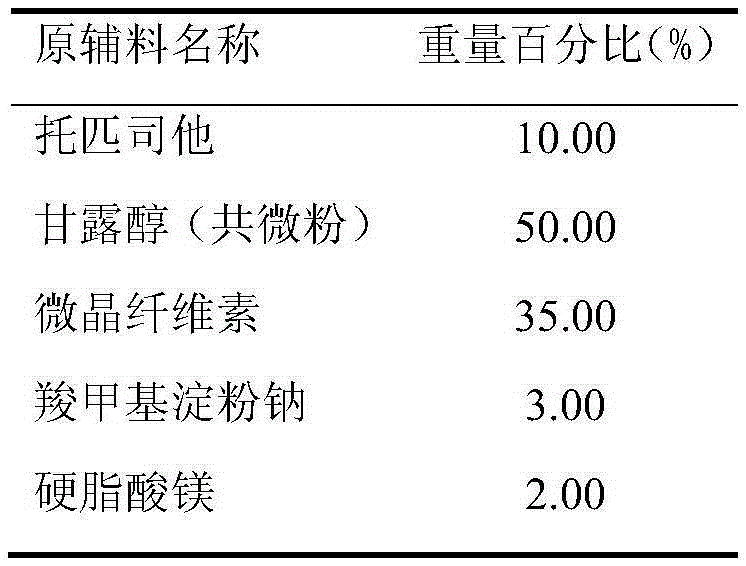
[0032] Prescription (Specification: 20mg)
[0033]
[0034] Preparation:
[0035] (1) Treatment of raw and auxiliary materials: the Dv90 of the co-micronized active ingredient topicastat and mannitol is 22.3 μm, and the remaining auxiliary materials are sieved through an 80-mesh sieve for later use;
[0036] (2) making soft material: after taking the co-micronized product of recipe quantity, microcrystalline cellulose, 2% carboxymethyl starch sodium and mixing evenly, make soft material with water;
[0037] (3) Granulation: get above-mentioned soft material and granulate with 18 mesh screens;
[0038] (4) Drying: dry the wet granules, control the temperature of the material at 50±5°C, and make the moisture of the granules reach about 2%;
[0039] (5) Grain sizing: the dried granules are sieved with a 24-mesh sieve;
[0040] (6) Total blending: the granulated granules are added to the converted magnesium stearate and 1% sodium carboxymethyl starch and mixed evenly. The mi...
Embodiment 2
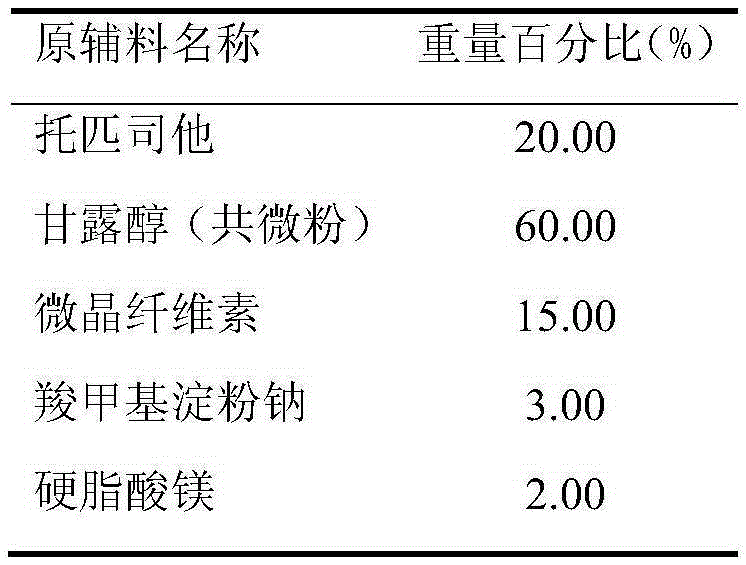
[0043] Prescription (Specification: 40mg)
[0044]
[0045] Preparation:
[0046] (1) Treatment of raw and auxiliary materials: the Dv90 of the co-micronized active ingredient topicastat and mannitol is 19.5 μm, and the remaining auxiliary materials are passed through an 80-mesh sieve for later use;
[0047] (2) making soft material: after taking the co-micronized product of recipe quantity, microcrystalline cellulose, 2% carboxymethyl starch sodium and mixing evenly, make soft material with water;
[0048] (3) Granulation: get above-mentioned soft material and granulate with 18 mesh screens;
[0049] (4) Drying: dry the wet granules, control the temperature of the material at 50±5°C, and make the moisture of the granules reach about 2%;
[0050] (5) Grain sizing: the dried granules are sieved with a 24-mesh sieve;
[0051] (6) Total blending: the granulated granules are added to the converted magnesium stearate and 1% sodium carboxymethyl starch and mixed evenly. The mi...
Embodiment 3
[0054] Prescription (Specification: 60mg)
[0055]
[0056]
[0057] Preparation:
[0058] (1) Treatment of raw and auxiliary materials: the Dv90 of the co-micronized active ingredient topicastat and mannitol is 10.3 μm, and the rest of the auxiliary materials are passed through an 80-mesh sieve for later use;
[0059] (2) making soft material: after taking the co-micronized product of recipe quantity, microcrystalline cellulose, 2.13% sodium carboxymethyl starch and mixing evenly, make soft material with water;
[0060] (3) Granulation: get above-mentioned soft material and granulate with 18 mesh screens;
[0061] (4) Drying: dry the wet granules, control the temperature of the material at 50±5°C, and make the moisture of the granules reach about 2%;
[0062] (5) Grain sizing: the dried granules are sieved with a 24-mesh sieve;
[0063](6) Total blending: the granulated granules are added to the converted magnesium stearate and 1.07% sodium carboxymethyl starch and m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com