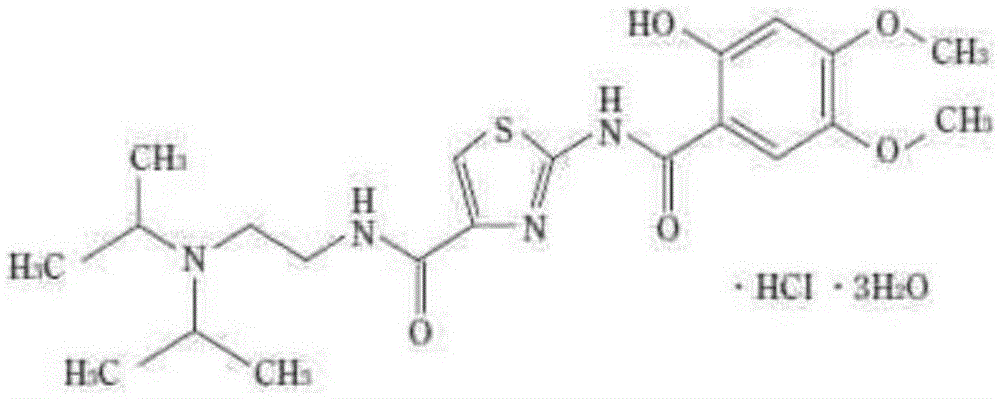
Acotiamide hydrochloride dispersible tablet and preparation method thereof
A technology of acotiamide hydrochloride and dispersible tablets, applied in the direction of dispersion liquid delivery, pill delivery, digestive system, etc., can solve the problems of difficult treatment for patients, influence on drug absorption, low dissolution rate, etc., and achieve good drug safety and human body Good absorption and convenient use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
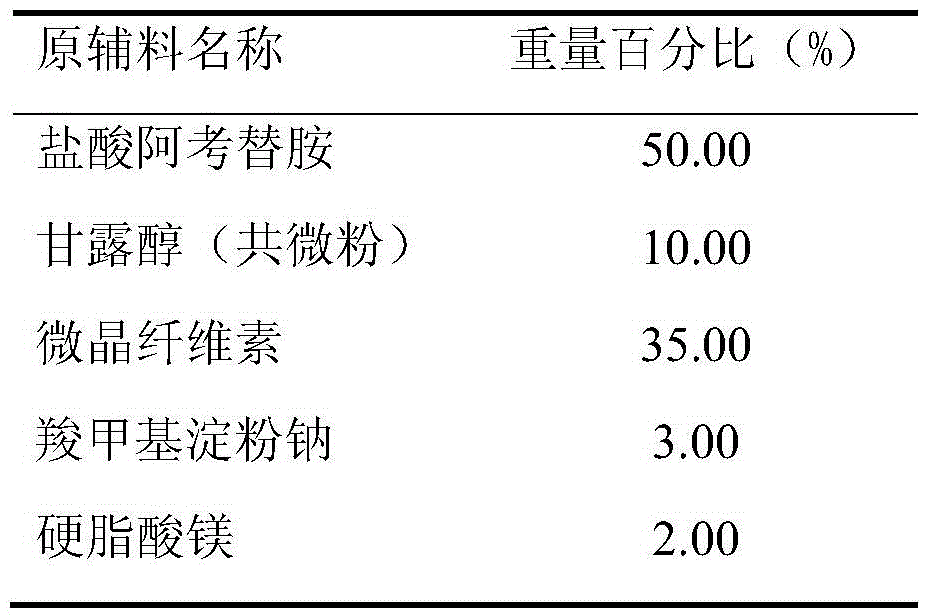
[0032] Prescription (Specification: 100mg)
[0033]
[0034] Preparation:
[0035] (1) Treatment of raw and auxiliary materials: the Dv90 of the co-micronized active ingredient acotiamide hydrochloride and mannitol is 21.4 μm, and the rest of the auxiliary materials are sieved through an 80-mesh sieve for later use;
[0036] (2) making soft material: after taking the co-micronized product of recipe quantity, microcrystalline cellulose, 2% carboxymethyl starch sodium and mixing evenly, make soft material with water;
[0037] (3) Granulation: get above-mentioned soft material and granulate with 18 mesh screens;
[0038] (4) Drying: dry the wet granules, control the temperature of the material at 50±5°C, and make the moisture of the granules reach about 2%;
[0039] (5) Grain sizing: the dried granules are sieved with a 24-mesh sieve;
[0040] (6) Total blending: the granulated granules are added to the converted magnesium stearate and 1% sodium carboxymethyl starch and mix...
Embodiment 2
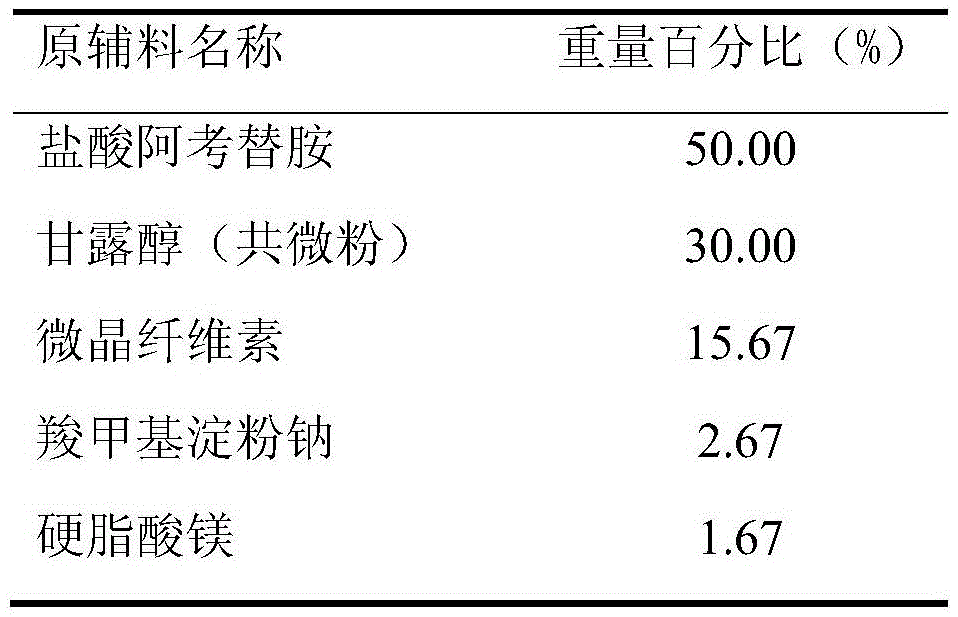
[0043] Prescription (Specification: 150mg)
[0044]
[0045] Preparation:
[0046] (1) Treatment of raw and auxiliary materials: the Dv90 of the co-micronized active ingredient acotiamide hydrochloride and mannitol is 18.6 μm, and the rest of the auxiliary materials are passed through an 80-mesh sieve for later use;
[0047] (2) making soft material: after taking the co-micronized product of recipe quantity, microcrystalline cellulose, 2% carboxymethyl starch sodium and mixing evenly, make soft material with water;
[0048] (3) Granulation: get above-mentioned soft material and granulate with 18 mesh screens;
[0049] (4) Drying: dry the wet granules, control the temperature of the material at 50±5°C, and make the moisture of the granules reach about 2%;
[0050] (5) Grain sizing: the dried granules are sieved with a 24-mesh sieve;
[0051] (6) Total blending: the granules after the granulation are added into the converted magnesium stearate and 0.67% sodium carboxymethy...
Embodiment 3
[0054] Prescription (Specification: 200mg)
[0055]
[0056]
[0057] Preparation:
[0058] (1) Treatment of raw and auxiliary materials: the Dv90 of the co-micronized active ingredient acotiamide hydrochloride and mannitol is 10.3 μm, and the rest of the auxiliary materials are passed through an 80-mesh sieve for later use;
[0059] (2) making soft material: after taking the co-micronized product of recipe quantity, microcrystalline cellulose, 2.22% carboxymethyl starch sodium and mixing evenly, make soft material with water;
[0060] (3) Granulation: get above-mentioned soft material and granulate with 18 mesh screens;
[0061](4) Drying: dry the wet granules, control the temperature of the material at 50±5°C, and make the moisture of the granules reach about 2%;
[0062] (5) Grain sizing: the dried granules are sieved with a 24-mesh sieve;
[0063] (6) Total blending: the granulated granules are added into the converted magnesium stearate and 1.11% sodium carboxyme...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com