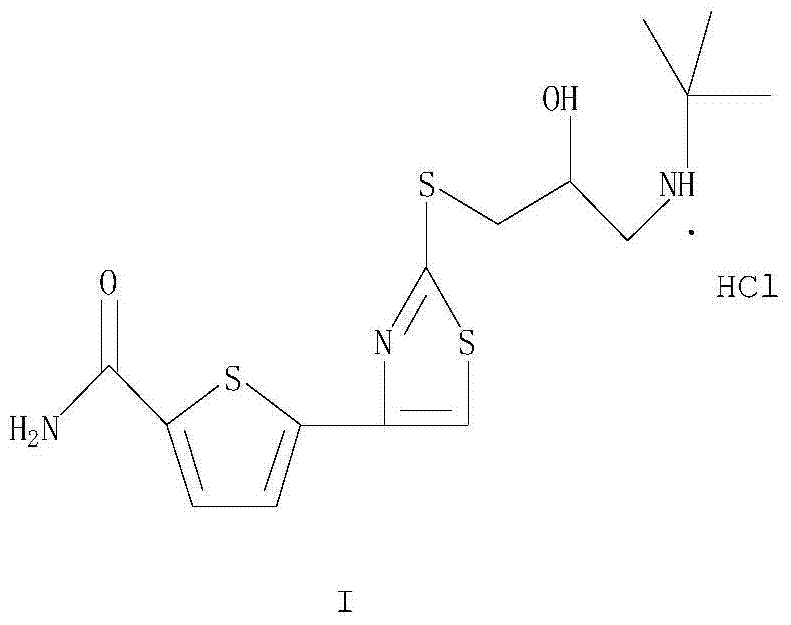
A new method for the preparation of alololol hydrochloride
A technology based on alololol hydrochloride and thiazolyl, which is applied in the field of medicine, can solve the problems of high cost of industrial scale-up, poor sample color, troublesome operation, etc., and achieve the effect of low cost, small impurities and easy control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
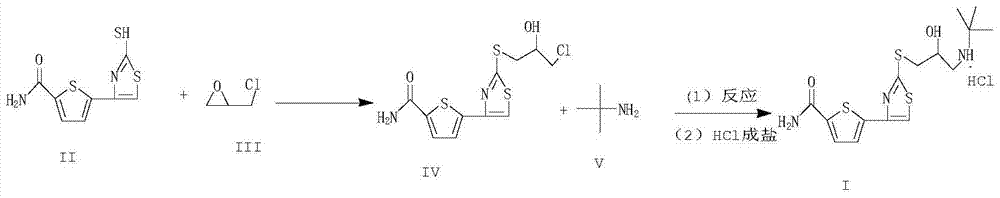
[0033] Example 1: Preparation of 5-[2-[(3-chloro-2-hydroxypropyl)thio]-4-thiazolyl]-2-thiophenecarboxamide
[0034] In a 250ml three-necked reaction flask, add 10g (41.26mmol) of 5-(2-mercapto-4-thiazolyl)-2-thiophene carboxamide (compound II), 4.1g (44.32mmol) of epichlorohydrin, anhydrous chlorine Zinc chloride 1.5g, anhydrous methanol 200ml, under stirring conditions, heat up to 65±3°C, control the temperature and react for about 3 hours, monitor with TLC spot plate, until the reaction is complete, cool down to room temperature naturally, filter, and concentrate the filtrate under reduced pressure To dryness, the intermediate compound 5-[2-[(3-chloro-2-hydroxypropyl)sulfanyl]-4-thiazolyl]-2-thiophenecarboxamide 11.23g was obtained, the yield was 81.27%, and the purity was checked by HPLC is 99.07%.
Embodiment 2
[0035] Example 2: Preparation of 5-[2-[(3-chloro-2-hydroxypropyl)thio]-4-thiazolyl]-2-thiophenecarboxamide
[0036] In a 250ml three-necked reaction flask, add 5-(2-mercapto-4-thiazolyl)-2-thiophene carboxamide (compound II) 10g (41.26mmol), epichlorohydrin 3.8g (41.26mmol), anhydrous chlorine Zinc chloride 2.0g, anhydrous methanol 180ml, under stirring conditions, heat up to 65±3°C, control the temperature and react for about 3 hours, monitor with TLC spot plate, until the reaction is complete, cool down to room temperature naturally, filter, and concentrate the filtrate under reduced pressure To dryness, the intermediate compound 5-[2-[(3-chloro-2-hydroxypropyl)sulfanyl]-4-thiazolyl]-2-thiophenecarboxamide 10.82g was obtained, the yield was 78.23%, and the purity was checked by HPLC is 99.15%.
Embodiment 3
[0037] Example 3: 5-[2-[[[3-(1,1-dimethylethyl)amino]-2-hydroxypropyl]thio]-4-thiazolyl]-2-thiophene carboxamide salt salt (alololol hydrochloride) is prepared
[0038] In a 250ml three-necked reaction flask, add the intermediate compound 5-[2-[(3-chloro-2-hydroxypropyl)sulfanyl]-4-thiazolyl]-2-thiophenecarboxamide 10g (29.86mmol), acetonitrile 150ml, stir at a medium speed, add 22.92g (298.60mmol) of tert-butylamine dropwise at room temperature, the dropwise addition is completed in about 30 minutes, then heat up to 60°C, control the temperature at 60±3°C for 10 hours, monitor with TLC spotting until the reaction is complete , naturally cool down to room temperature, adjust the pH of the system to 1~2 with 15% HCl, then use an ice-water bath to control the temperature T=0~5°C, stir and crystallize for 3 hours, filter with suction, wash the filter cake with 20ml of methanol, and drain it, 50 ℃ vacuum drying to obtain 10.2 g of the crude product of arololol hydrochloride as a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com