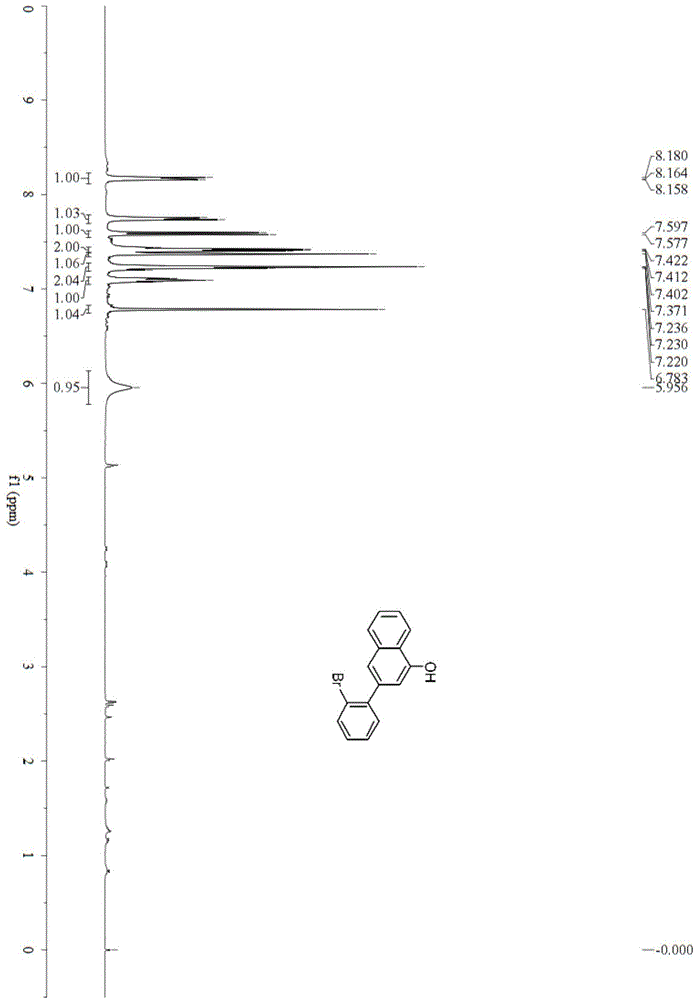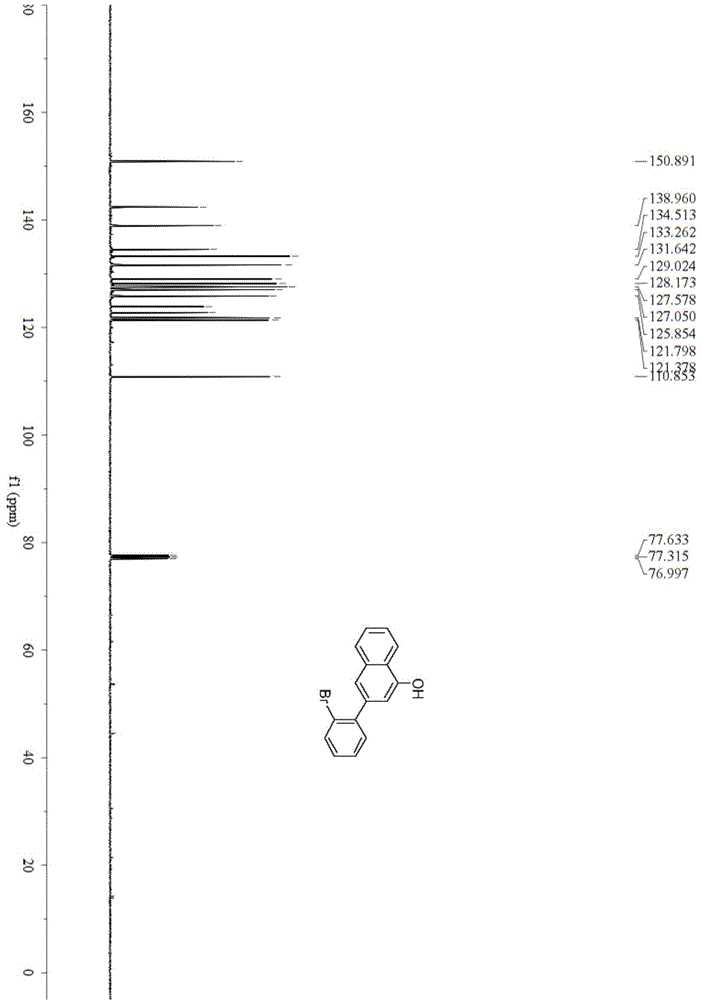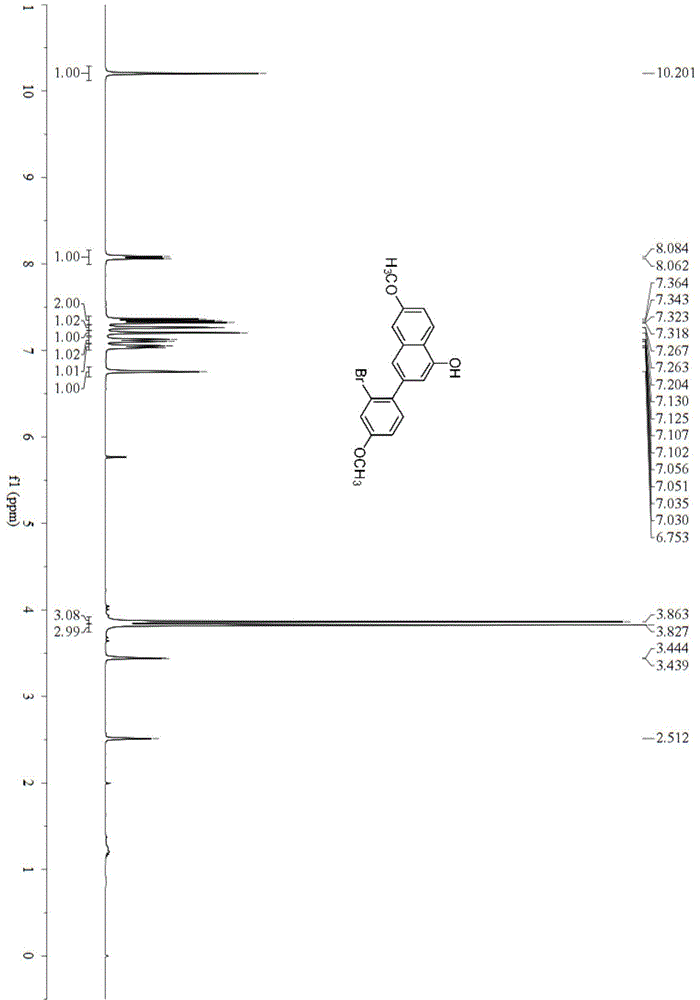Preparation method of alpha-naphthol compound
A compound, naphthol technology, applied in pharmaceutical and chemical intermediates and related chemical fields, can solve problems such as complex starting materials, harsh reaction conditions, and application restrictions, and achieve the effect of easy-to-obtain, simple raw materials, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0039] Example 1: Synthesis of 3-(2-bromophenyl)naphthalen-1-ol(1a)
[0040]
[0041] In a 25mL reactor, add potassium tert-butoxide (0.112g, 1mmol) and copper acetate (0.010g, 0.050mmol). After nitrogen replacement for 3 times, add 0.40mL of anhydrous tetrahydrofuran under nitrogen protection, and add the adjacent Bromoacetophenone (0.198g, 1mmol), stirred at 30°C for 5h, column chromatography (silica gel, 200-300 mesh; developing solvent, petroleum ether: ethyl acetate = 20:1) to obtain 3-(2-bromophenyl)naphthalen -1-ol0.119g, the yield is 80%. Pale yellow oil; IR(neat)ν3396,3054,1702,1599,1257,1019,911,849,758,531cm -1 ; 1 H-NMR (400MHz, CDCl 3 )δ5.96(s,1H), 6.78(s,1H), 7.07–7.11(m,1H), 7.20–7.25(m,2H), 7.37(d,J=5.2Hz,1H),7.42(dd ,J=8.0,4.0Hz,2H),7.59(d,J=8.0Hz,1H),7.73-7.76(m,1H); 13 C-NMR (100MHz, CDCl 3 )δ110.9,121.4,121.8,122.8,123.9,125.9,127.1,127.6,128.2,129.0,131.6,133.3,134.5,139.0,142.4,150.9; HRMS(EI)calcd for C 16 H 21 BrO:297.9993[M] + ;Found:298.0000.
Example Embodiment
[0042] Example 2: Synthesis of 3-(2-bromo-4-methoxyphenyl)-6-methoxynaphthalen-1-ol(2a)
[0043]
[0044] In a 25mL reactor, add sodium tert-butoxide (1.920g, 20mmol) and cuprous oxide (0.016g, 0.100mmol). After nitrogen replacement for 3 times, add anhydrous N,N-dimethylformate under nitrogen protection Amide 3.40mL, add 4-methoxy-2-bromoacetophenone (0.228g, 1mmol) with stirring, stir at 0℃ for 5h, column chromatography (silica gel, 200-300 mesh; developing solvent, petroleum ether: ethyl acetate Ester=20:1) to obtain 0.116 g of 3-(2-bromo-4-methoxyphenyl)-6-methoxynaphthalen-1-ol with a yield of 65%. Mp105.0–105.2℃; IR(neat)ν3424,1634,1605,1394,1220,1024,813,574cm -1 ; 1 H-NMR(d 6 -DMSO,400MHz)δ3.83(s,3H), 3.86(s,3H), 6.75(s,1H), 7.04(dd,J=8.4,2.0Hz,1H), 7.12(dd,J=9.2, 2.0Hz, 1H), 7.20 (s, 1H), 7.26 (d, J = 1.6 Hz, 1H), 7.34 (dd, J = 13.2, 5.3 Hz, 2H), 8.07 (d, J = 8.8 Hz, 1H) 10.20(s,1H); 13 C-NMR(d 6 -DMSO,100MHz)δ55.6,56.1,106.5,108.5,114.3,117.6,118.4,118.5,119.3,122.5,1...
Example Embodiment
[0045] Example 3: Synthesis of 3-(2-bromo-4-fluorophenyl)-6-fluoronaphthalen-1-ol(3a)
[0046]
[0047] In a 25mL reactor, add sodium tert-butoxide (1.920g, 20mmol) and cuprous oxide (0.032g, 0.200mmol). After nitrogen replacement for 3 times, add anhydrous N,N-dimethylformaldehyde under nitrogen protection. Amide 3.40mL, add 4-fluoro-2-bromoacetophenone (0.217g, 1mmol) under stirring, stir at 0°C for 5h, column chromatography (silica gel, 200-300 mesh; developing solvent, petroleum ether: ethyl acetate = 20:1) 0.104 g of 3-(2-bromo-4-fluorophenyl)-6-fluoronaphthalen-1-ol was obtained, and the yield was 62%. Mp99.0-99.4℃; IR(neat)ν3073,1581,1489,1397,1025,859,820,759cm -1 ; 1 H-NMR(d 6 -DMSO,400MHz)δ6.59(s,1H), 6.94-7.02(m,3H), 7.09-7.13(m,1H), 7.25-7.31(m,2H), 7.90-7.94(m,1H), 10.27(s,1H); 13 C-NMR(d 6 -DMSO,100MHz)δ109.6,111.1(d,J C-F =20.4Hz),115.2,115.5(d,J C-F =8.2Hz),118.7(d,J C-F =4.9Hz), 120.3(d, J C-F =24.2Hz),121.5,122.5(d,J C-F =9.7Hz),125.5(d,J C-F =9.2Hz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com