Creatinine derivatives, creatinine immunogens and their specific antibodies, and creatinine detection kits
An immunogen and derivative technology, applied in the field of immunodetection, can solve the problems of inability to perform automated analysis, low sensitivity, poor creatinine stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
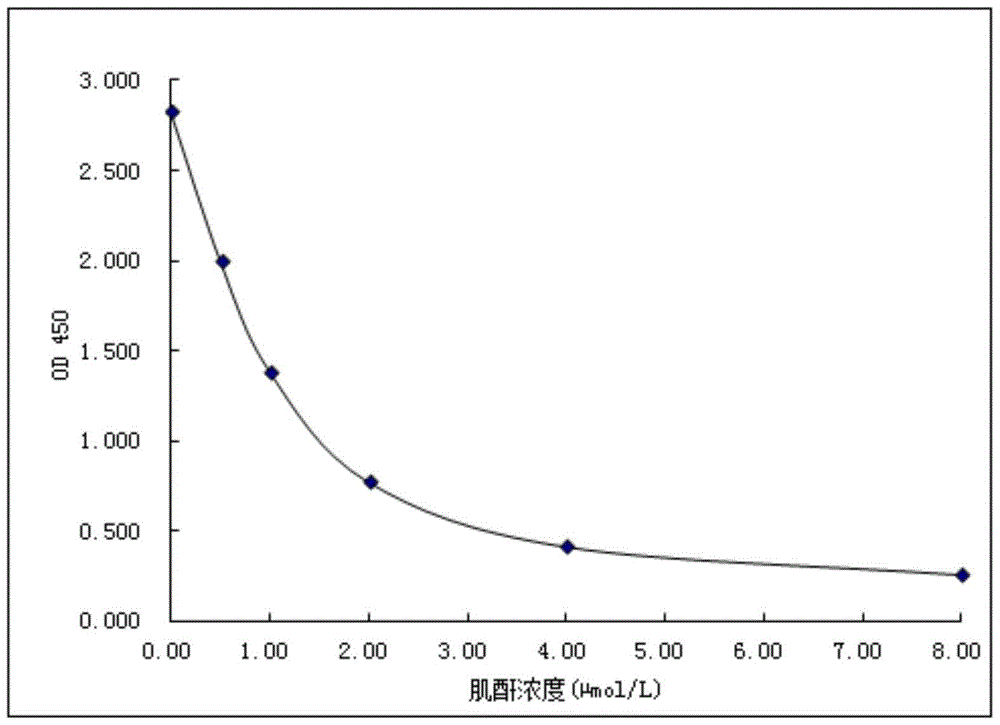
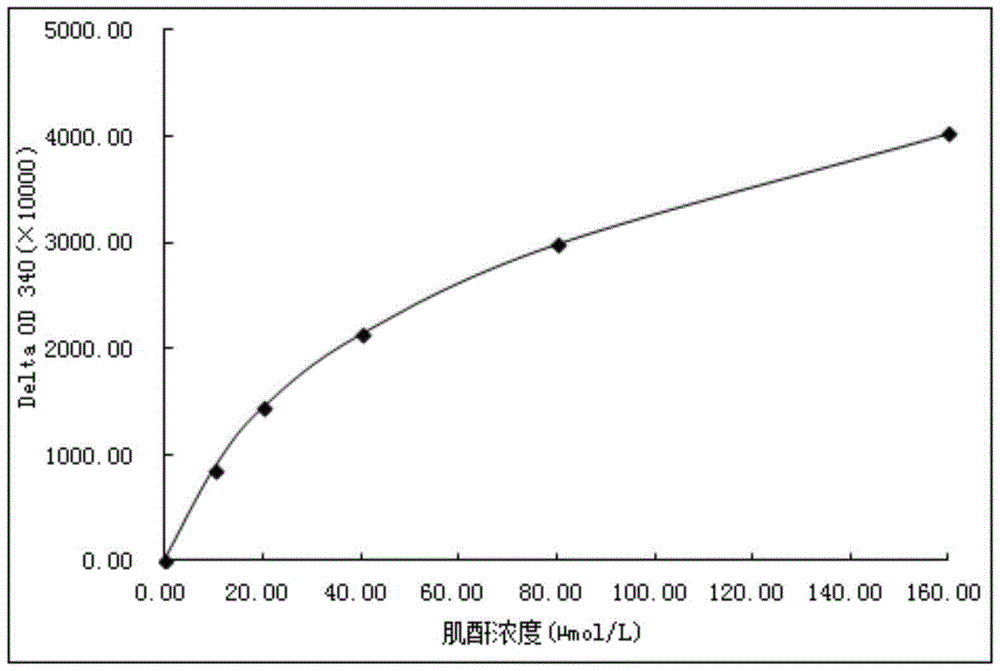
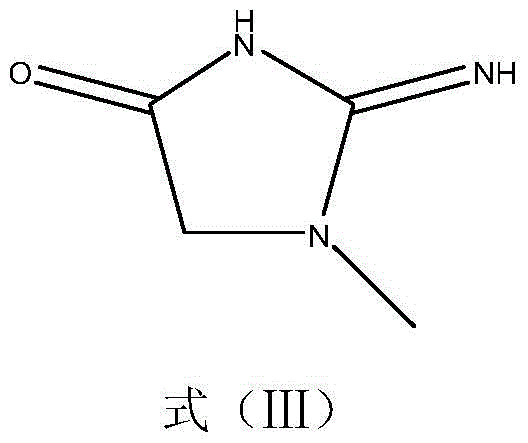
Image
Examples
Embodiment 1
[0038] Example 1: Synthesis and structure confirmation of creatinine derivatives
[0039] The chemical structure of the creatinine derivative used in the following examples is shown in formula (IV):
[0040]
[0041]
[0042] The synthetic route of the creatinine derivative shown in formula (IV) is as follows:
[0043]
[0044] The specific synthetic steps of the creatinine derivative shown in formula (IV) are as follows:
[0045] (1) Synthesis of compound 2
[0046]
[0047] 1) Weigh 10.0g (88.4mmol) of compound 1 and 17.3g (88.4mmol) of tert-butyl bromoacetate and dissolve them together in 100mL of dimethylformamide (DMF), then heat the solution to 85°C overnight. To this solution was added 350 mL of ethyl acrylate (EA), and the reaction mixture was stirred at 0° C. for 15 min. The solid precipitate in the solution was filtered out, washed with acetone and then vacuum-dried to finally obtain 5.00 g of compound 2 as a white solid with a yield of 25%.
[0048] ...
Embodiment 2
[0055] Embodiment 2: When n=2, the preparation steps of creatinine derivatives:
[0056] (1)2-(CH 2 ) 2 Synthesis of -COO-tert-Butyl Creatinine
[0057] 1) Weigh 12.0g (106.8mmol) of compound 1 and 18.5g (88.4mmol) of tert-butyl bromopropionate and dissolve them together in 100mL of dimethylformamide (DMF), then heat the solution to 80°C overnight. To this solution was added 350 mL of ethyl acrylate (EA), and the reaction mixture was stirred at 2° C. for 20 min. The solid precipitate in the solution was filtered out, washed with acetone and then vacuum-dried to finally obtain 5.10 g of a white solid intermediate product with a yield of 25.5%.
[0058] 2) Using Varian III plus 300MHz to scan the NMR spectrum of the above compound, using TMS as an internal standard. The results were characterized as 2-(CH 2 ) 2 -COO-tert-butyl creatinine.
[0059] (2) Synthesis of creatinine derivatives
[0060] 1) Weigh 2.00g (8.8mmol) of the above 2-(CH 2 ) 2 -COO-tert-butyl creatini...
Embodiment 3
[0064] Embodiment 3: When n=3, the preparation steps of creatinine derivatives:
[0065] (1)2-(CH 2 ) 3 Synthesis of -COO-tert-Butyl Creatinine
[0066]1) Weigh 12.0g (88.4mmol) of compound 1 and 19.7g (88.4mmol) of tert-butyl bromobutyrate and dissolve them together in 120mL of dimethylformamide (DMF), then heat the solution to 90°C overnight. To this solution was added 350 mL of ethyl acrylate (EA), and the reaction mixture was stirred at 4 °C for 25 min. The solid precipitate in the solution was filtered out, washed with acetone and then vacuum-dried to finally obtain 5.05 g of compound 2 as a white solid with a yield of 24%.
[0067] 2) Using Varian III plus 300MHz to scan the NMR spectrum of the above compound, using TMS as an internal standard. The results were characterized by the compound shown as 2-(CH2)3-COO-tert-butyl creatinine.
[0068] (2) Synthesis of creatinine derivatives
[0069] 1) Weigh 2.00 g (8.8 mmol) of compound 2, dissolve it in 20 mL of HCl (1 M...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com