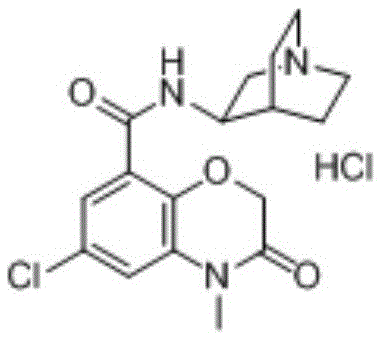
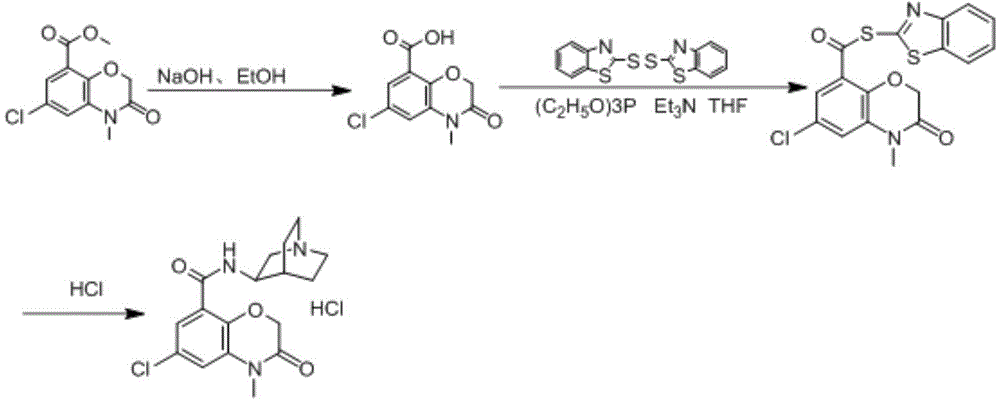
A kind of preparation method of azasetron hydrochloride
A technology of azasetron hydrochloride and aminoquinuclidine hydrochloride, which is applied in the field of medicine, can solve problems such as difficult removal of by-products, environmental and equipment pollution, unfavorable production methods, etc., to achieve mild reaction conditions, avoid pollution, and yield high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] In a 500ml three-necked flask, add 150ml of water and 12g (0.3mol) of sodium hydroxide, stir until the solid is completely dissolved, then add 100ml of ethanol, cool down to 20°C, add 6-chloro-4-methyl-3,4-di Hydrogen-3-oxo-2H-1,4-benzoxazine-8-carboxylic acid methyl ester 25.5g (0.1mol), stirring reaction for 3h, the reaction is completed, cooling below 10°C, dilute hydrochloric acid to adjust the pH value to 2 , filtered, and dried to obtain off-white solid of 6-chloro-4-methyl-3,4-dihydro-3-oxo-2H-1,4-benzoxazine-8-carboxylic acid, 21.9g, yield The rate is 91%.
[0081] 6-chloro-4-methyl-3,4-dihydro-3-oxo-2H-1,4-benzoxazine-8-carboxylic acid (12.0g, 0.05mol), triethyl phosphite ( Add 10.0g, 0.062mol), 2,2'-dithiodibenzothiazole (20.0g, 0.062mol) and tetrahydrofuran (100ml) into a dry reaction flask, stir at room temperature for 1h, and control the temperature in an ice-water bath below 20°C. A solution of triethylamine (8.6ml) in tetrahydrofuran (20ml) was added dr...
Embodiment 2
[0087]In a 500ml three-neck flask, add 200ml of water and 12g (0.3mol) of sodium hydroxide, stir until the solid is completely dissolved, then add 100ml of ethanol, cool down to 21°C, add 6-chloro-4-methyl-3,4-di Hydrogen-3-oxo-2H-1,4-benzoxazine-8-carboxylic acid methyl ester 25.5g (0.1mol), stirred for 3h, the reaction was completed, cooled below 10°C, dilute hydrochloric acid to adjust the pH value to 1 ~2, filtered and dried to give off-white solid of 6-chloro-4-methyl-3,4-dihydro-3-oxo-2H-1,4-benzoxazine-8-carboxylic acid, 20.9g , yield 86.7%.
[0088] 6-chloro-4-methyl-3,4-dihydro-3-oxo-2H-1,4-benzoxazine-8-carboxylic acid (12.0g, 0.05mol), triethyl phosphite ( Add 9.7g, 0.06mol), 2,2'-dithiodibenzothiazole (19.4g, 0.06mol) and tetrahydrofuran (100ml) into a dry reaction flask, stir at room temperature for 1h, and control the temperature in an ice-water bath below 20°C. A solution of triethylamine (8.6ml) in tetrahydrofuran (20ml) was added dropwise. After dropping, r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com