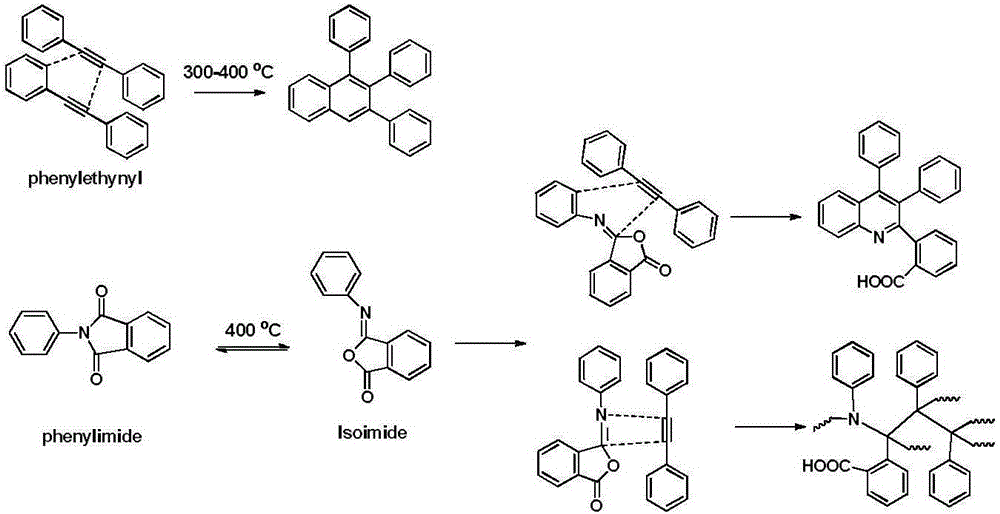
Monomer containing phenylimide phenylacetylene structure and high temperature self-crosslinking copolyester and preparation method thereof
A technology containing benzimide phenylacetylene and copolyester, which is applied in the field of high-temperature self-crosslinking copolyester and its preparation, can solve the problems of limiting the application range of polyester and increasing smoke release, and achieve high self-crosslinking Combined flame retardant efficiency, flame retardant rate increase, anti-melt droplet smoke suppression effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] In a 250mL three-neck flask equipped with a nitrogen protection device, magnetic stirring, and a water trap equipped with a spherical condenser, add 10g of dimethyl 5-aminoisophthalate and 80mL of NMP. Under the protection of nitrogen, stir for 30 minutes to obtain a homogeneous solution, then add 11.9g of 4-phenylacetylene phthalic anhydride, heat the solution to 75°C, and keep it for 2h; then add 20g of toluene, the reaction is gradually heated to 180°C, and the generated water is passed through the water separator Take out; after reacting for 6-8 hours, cool to 120°C, pour the mixed solution into excess deionized water to obtain a yellow-green solid, filter it with suction, wash with water and dry. That is, dimethyl 5-(4-phenylacetylene benzimide)-1,3-dibenzoate was obtained.
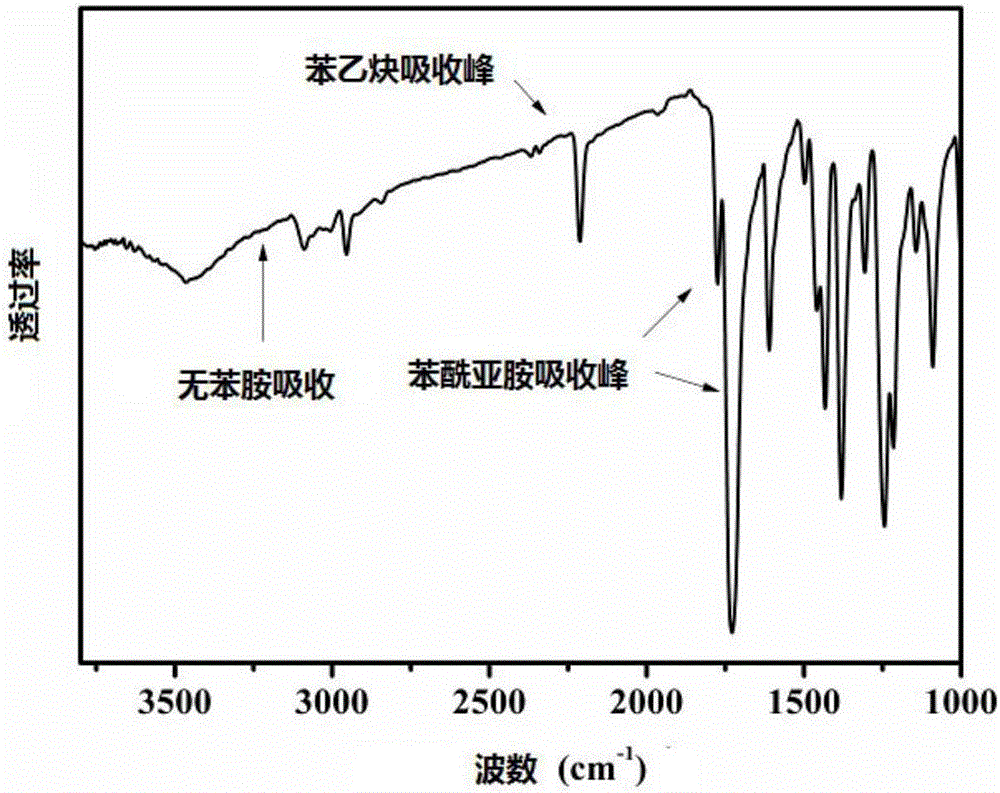
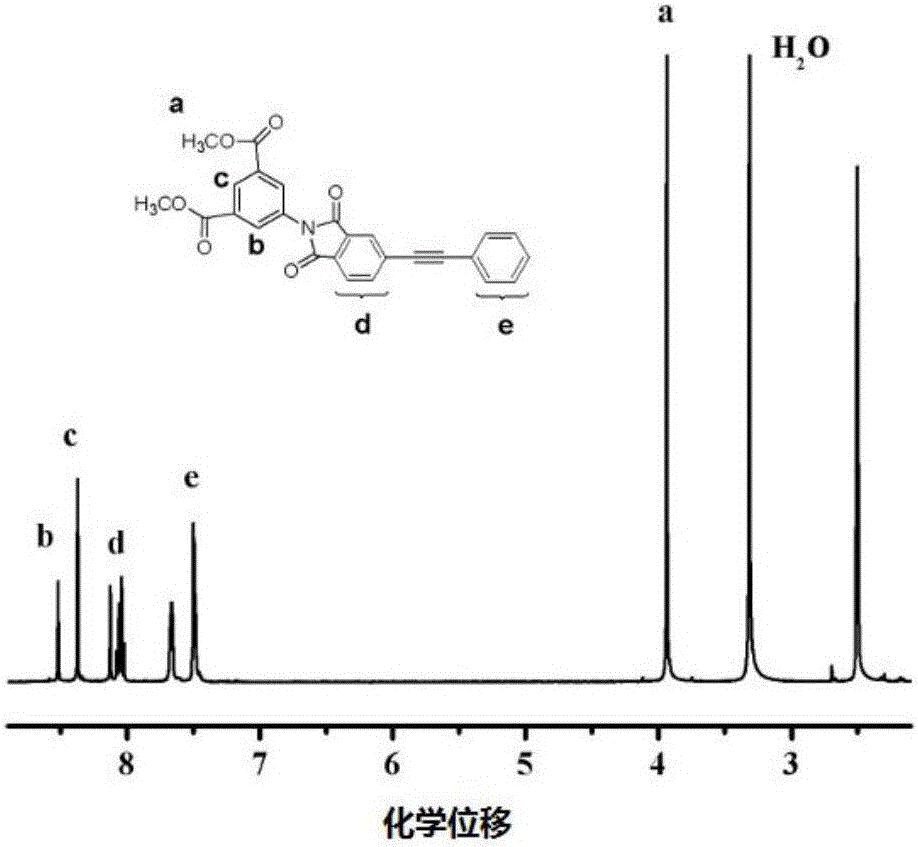
[0058]The infrared absorption characteristic peaks of the monomer are: 2920, 2853, 1780, 1722, 600, 1450cm -1 . Its NMR chemical shifts are: 8.2-8.6, 7.5-8.1, 3.9ppm. like figure 1 , fig...
Embodiment 2
[0060] Add 10 g of aniline and 80 mL of NMP to a 250 mL three-neck flask equipped with a nitrogen protection device, magnetic stirring, and a water trap equipped with a spherical condenser. Under the protection of nitrogen, stir for 30 minutes to obtain a homogeneous solution, then add 15g of 4-(4-acetylene phthalic anhydride)-1,2-dibenzoic acid, heat the solution to 75°C, and keep it for 2h; then add 20g of toluene, and the reaction is gradually heated to 180°C, the generated water is taken out by the water separator; after reacting for 6-8 hours, cool to 120°C, pour the mixed solution into excess deionized water, filter with suction, wash with water and dry. That is, 4-benzimide phenylacetylene-1,2-dibenzoic acid was obtained.
Embodiment 3
[0062] Add 10 g of aniline and 80 mL of NMP to a 250 mL three-neck flask equipped with a nitrogen protection device, magnetic stirring, and a water trap equipped with a spherical condenser. Under the protection of nitrogen, stir for 30 minutes to obtain a homogeneous solution, then add 15g of 5-(4-acetylene phthalic anhydride)-1,3-dibenzoic acid, heat the solution to 75°C and keep it for 2h; then add 20g of toluene, and the reaction temperature is gradually raised to 180 °C, the generated water is taken out by the water separator; after reacting for 6-8 hours, cool to 120 °C, pour the mixed solution into excess deionized water, filter with suction, wash with water and dry. That is, 5-benzimide phenylacetylene-1,3-dibenzoic acid was obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| peak heat release rate | aaaaa | aaaaa |
| release amount | aaaaa | aaaaa |
| peak heat release rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com