Biphenyl compounds, intermediates, preparation methods, pharmaceutical compositions and applications thereof
A compound, biphenyl technology, applied in the field of biphenyl compounds, can solve the problems of β-cell function damage in patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
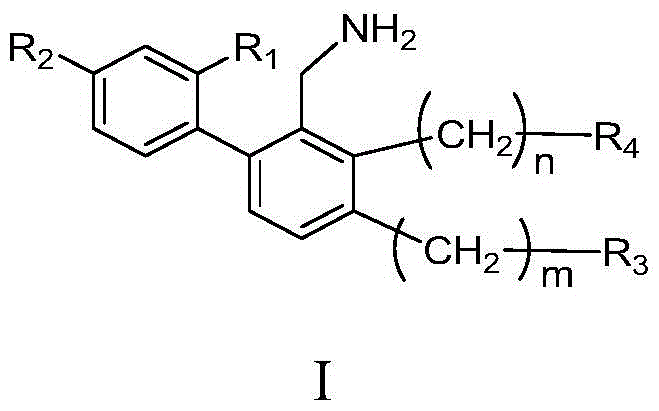
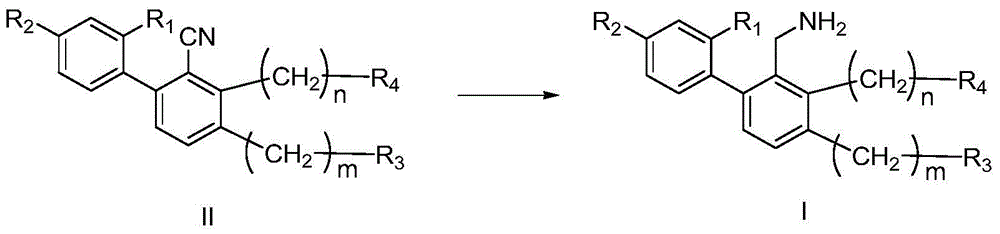
[0358] 2',4'-Dichloro-2-aminomethyl-3-(3-trifluoromethyl-5,6,7,8-tetrahydro-[1,2,4]triazol[4,3- a] preparation of pyrazin-7-yl) methyl biphenyl (I-1)
[0359] 2',4'-Dichloro-2-cyano-3-(3-trifluoromethyl-5,6,7,8-tetrahydro-[1,2,4]triazol[4,3-a ]pyrazin-7-yl)methylbiphenyl (0.50g, 1.1mmol), Raney Ni (0.10g) and 2mL NH 3 / EtOH (3mol / L) and 8ml EtOH were placed in a hydrogenation reaction kettle, hydrogen was passed through, the pressure was 0.4MPa, and the reaction was carried out at room temperature for 6h, the TLC raw material disappeared completely, the reaction was stopped, the mother liquor was concentrated by suction filtration, and an off-white solid was obtained by column chromatography 0.12g, yield 24.2%.
[0360] The synthesis method of I-2, I-3 and I-4 is the same as that of I-1, and the physical and chemical properties of I-1~I-4 are shown in Table 2.
Embodiment 2
[0362] Preparation of 2',4'-dichloro-2-aminomethyl-3-imidazolylmethylbiphenyl hydrochloride (I-6 hydrochloride)
[0363] 2',4'-dichloro-2-cyano-3-bromomethylbiphenyl (0.50g, 1.5mmol), imidazole (0.11g, 1.5mmol), dissolved in 5mL of acetonitrile, then added anhydrous potassium carbonate (0.61g, 4.4mmol), stirred at 25°C for 2h, the TLC raw material disappeared completely, stopped stirring, evaporated the solvent, and column chromatography (dichloromethane:methanol=60:1) gave the oily intermediate 2', 4' - 0.35 g of dichloro-2-cyano-3-imidazolylmethyl, yield 72.0%.
[0364] The above oil (0.35g, 1.1mmol), Raney Ni (0.07g) and 3mL NH 3 / EtOH (3mol / L) and 7mlEtOH were placed in a hydrogenation reaction kettle, hydrogen was passed through, the pressure was 0.4MPa, and the reaction was carried out at room temperature for 6h. The TLC raw material disappeared completely, and the reaction was stopped. Suction filtration, the mother liquor was concentrated to obtain 0.32g oil, and colu...
Embodiment 3
[0367] Preparation of 2',4'-dichloro-2-aminomethyl-4-(1,2,4-triazol-1-yl)methylbiphenyl hydrochloride (I-24 hydrochloride)
[0368] 2',4'-dichloro-2-cyano-4-bromomethylbiphenyl (0.50g, 1.5mmol), 1,2,4-triazole (0.11g, 1.6mmol), dissolved in 5mL acetonitrile , then added anhydrous potassium carbonate (0.61g, 4.4mmol), stirred at room temperature for 9h, the TLC raw material disappeared completely, stopped stirring, evaporated the solvent, and column chromatography (petroleum ether: ethyl acetate=1:1) gave an oily substance Intermediate 2',4'-dichloro-2-cyano-4-(1,2,4-triazol-1-yl)methylbiphenyl 0.45g, yield 93.4%, 1 H-NMR (CDCl 3 ), δ(ppm): 8.21(s,1H), 8.04(s,1H), 7.67(s,1H), 7.57-7.54(m,2H), 7.47-7.45(d,1H,J=7.6Hz) ,7.40-7.37(m,1H),7.29-7.26(d,1H,J=8.4Hz),5.46(s,2H)
[0369] 2',4'-Dichloro-2-cyano-4-(1,2,4-triazol-1-yl)methylbiphenyl (0.45g, 1.4mmol), Raney Ni (0.09g) and 3mL NH 3 / EtOH (3mol / L) and 7ml EtOH were placed in a hydrogenation reaction kettle, hydrogen was pas...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap