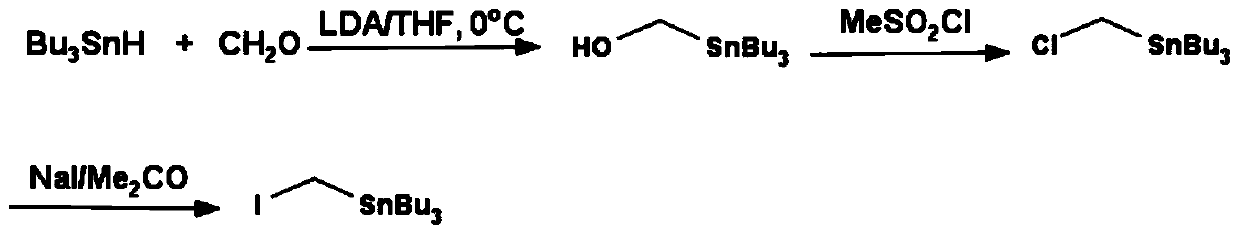
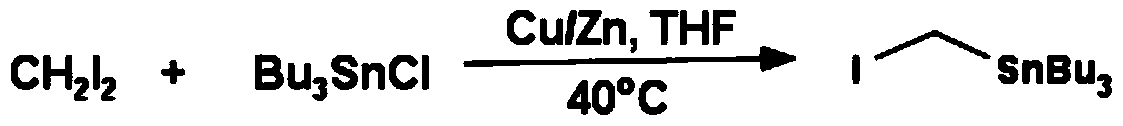
One-pot Synthesis of Iodomethyltributyltin
A technology of tributyl tin iodide and n-butyl lithium, applied in the direction of tin organic compounds and the like, can solve the problems of difficult industrial production, long reaction period, harsh conditions, etc., and achieves easy commercial production, low production cost, and high product quality. A stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] In a 1L three-necked reaction flask, add diiodomethane (26.7g, 0.1mol), tributyltin chloride (32.5g, 0.1mol) and 200mL of anhydrous tetrahydrofuran, under nitrogen protection, cool to -78°C, slowly drop Add n-butyllithium (42mL, 0.11mol, 2.5M in hexanes), after the addition is complete, slowly warm up to room temperature, react for 12h, evaporate the solvent under reduced pressure with a rotary evaporator, then extract the product with 200mL of n-hexane, filter to remove Insoluble matter, remove solvent with rotary evaporator, generate pale yellow liquid, then underpressure distillation (140-142 ℃ / 1mmHg) obtains colorless liquid 29.3g, productive rate 68%, 1HNMR (CD 3 Cl): 1.94ppm, unimodal (2H); 1.56ppm, multimodal (6H); 1.46ppm, multimodal (6H); 1.05ppm, multimodal (6H); 0.97ppm, multimodal (9H).
Embodiment 2
[0031] In a 1L three-necked reaction flask, add diiodomethane (26.7g, 0.1mol), tributyltin chloride (32.5g, 0.1mol) and 200mL of anhydrous ether, under nitrogen protection, cool to -78°C, slowly drop Add n-butyllithium (42mL, 0.11mol, 2.5M in hexanes), after the addition is complete, slowly warm up to room temperature, react for 12h, evaporate the solvent under reduced pressure with a rotary evaporator, then extract the product with 200mL of n-hexane, filter to remove Insoluble matter, remove solvent with rotary evaporator, generate pale yellow liquid, then underpressure distillation (140-142 ℃ / 1mmHg) obtains colorless liquid 28g, productive rate 65%, 1HNMR (CD 3 Cl): 1.94ppm, unimodal (2H); 1.56ppm, multimodal (6H); 1.46ppm, multimodal (6H); 1.05ppm, multimodal (6H); 0.97ppm, multimodal (9H).
Embodiment 3
[0033] In a 10L three-necked reaction flask, add diiodomethane (1000g, 3.73mol), tributyltin chloride (1215.3g, 3.73mol) and 2L anhydrous tetrahydrofuran, under nitrogen protection, cool to -78°C, slowly drop n-Butyllithium (1567mL, 3.91mol, 2.5M in hexanes), after the addition, slowly warmed up to room temperature, reacted for 12h, evaporated the solvent with a rotary evaporator under reduced pressure, then extracted the product with 3L of n-hexane, filtered to remove the insoluble Substance, remove solvent with rotary evaporator, generate pale yellow liquid, then underpressure distillation (140-142 ℃ / 1mmHg) obtains colorless liquid 1012g, productive rate 63%, 1HNMR (CD 3 Cl): 1.94ppm, unimodal (2H); 1.56ppm, multimodal (6H); 1.46ppm, multimodal (6H); 1.05ppm, multimodal (6H); 0.97ppm, multimodal (9H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com