A method for preparing (2s, 3r)-2-benzamidomethyl-3-hydroxybutyric acid methyl ester
A technology of benzamidomethyl and methyl hydroxybutyrate, which is applied in the field of biopharmaceuticals, can solve the problems of reducing catalytic efficiency, affecting AdhR catalytic effect, increasing the complexity of product separation and purification, and achieving the goal of improving conversion rate and purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Construction of embodiment 1 plasmid pET30-LbADH
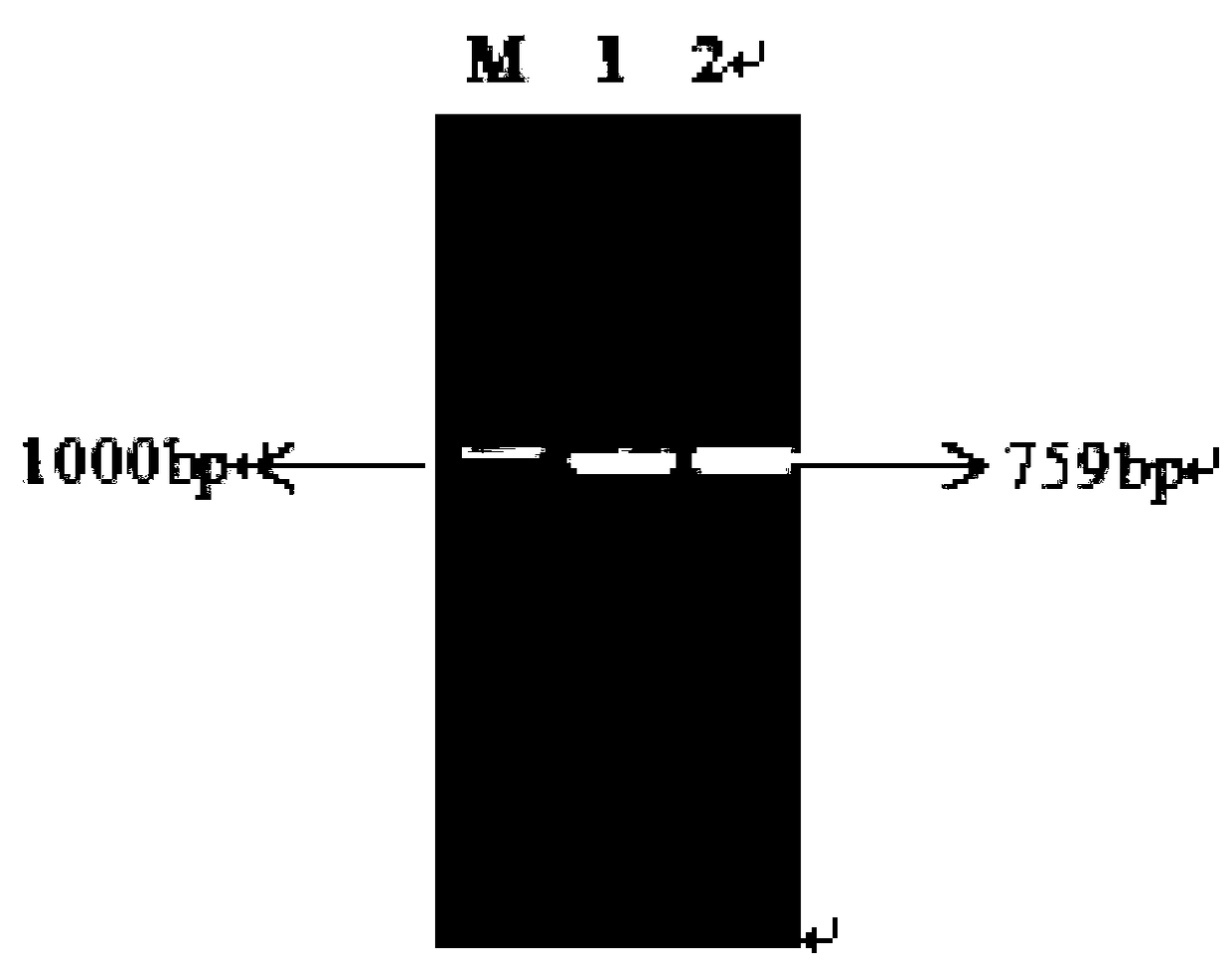
[0045] The Lbadh gene was cloned with primers F_LbADH / R_LbADH to obtain a 759bp Lbadh gene (as shown in SEQ ID NO.1). Nucleic acid electrophoresis to verify gene size, such as figure 1 .
[0046] The sequence of primer F_LbADH is: 5'CGC GGATCC ATGTCTAACCGTTTGGATG-3';
[0047] The sequence of primer R_LbADH is: 5'CCG CTCGAG CTATTGAGCAGTGTAGCCAC-3'.
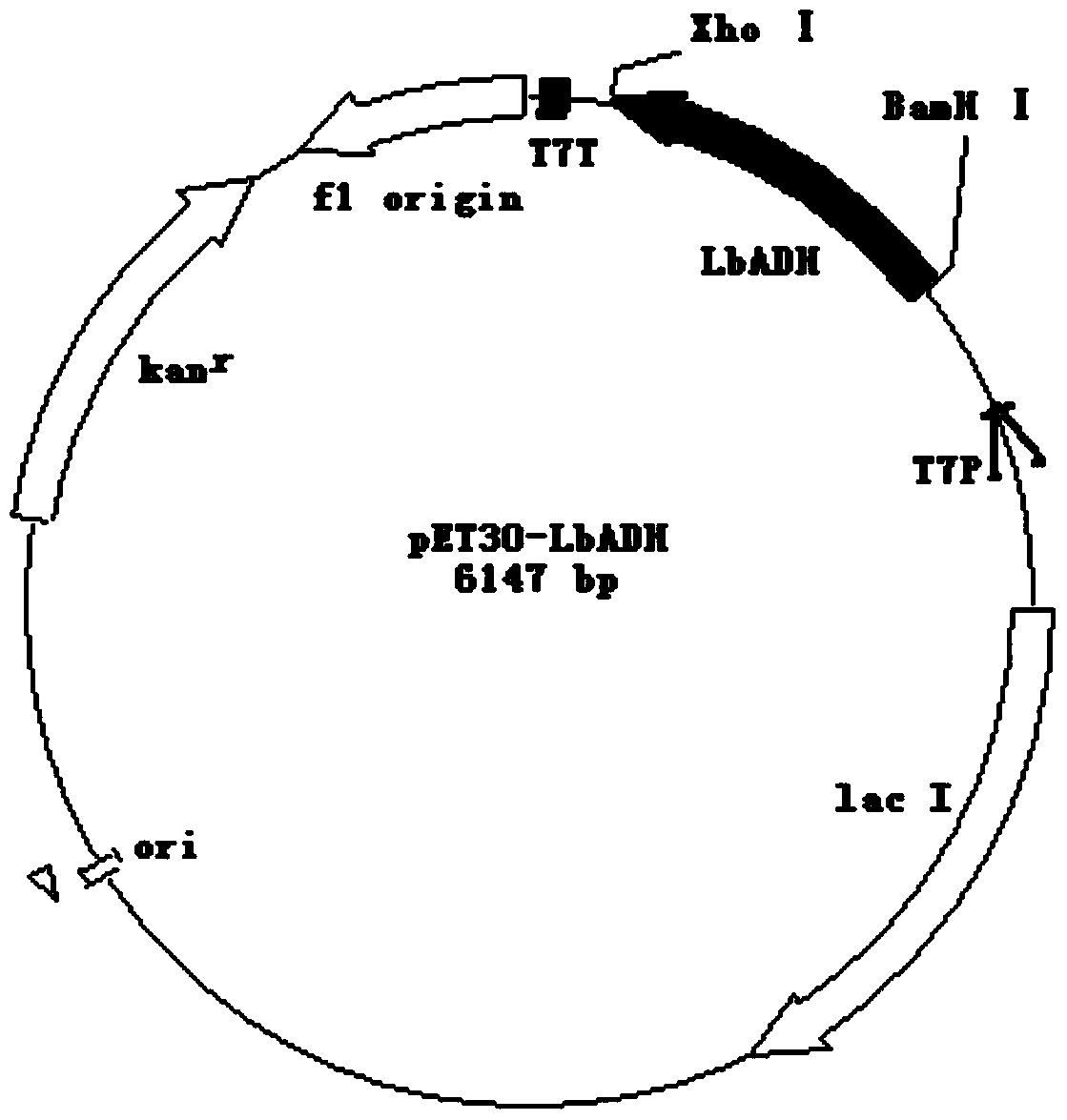
[0048] BamHI and XhoI double-digest Lbadh gene, recover the gene band after digestion, BamHI and XhoI double-digest pET-30a(+) plasmid, recover the plasmid band after digestion, and digest the Lbadh gene and enzyme The excised pET-30a(+) plasmid was ligated with ligase and transformed into the cloning host Escherichia coli DH5α. Colony PCR was performed with primers F_LbADH / R_LbADH to verify the transformed recombinant, and then the recombinant plasmid was extracted for sequencing. The recombinant plasmid with correct sequencing results is the recombinant plasmid pE...
Embodiment 2
[0049] Construction of embodiment 2 plasmid pET30-GdhBM
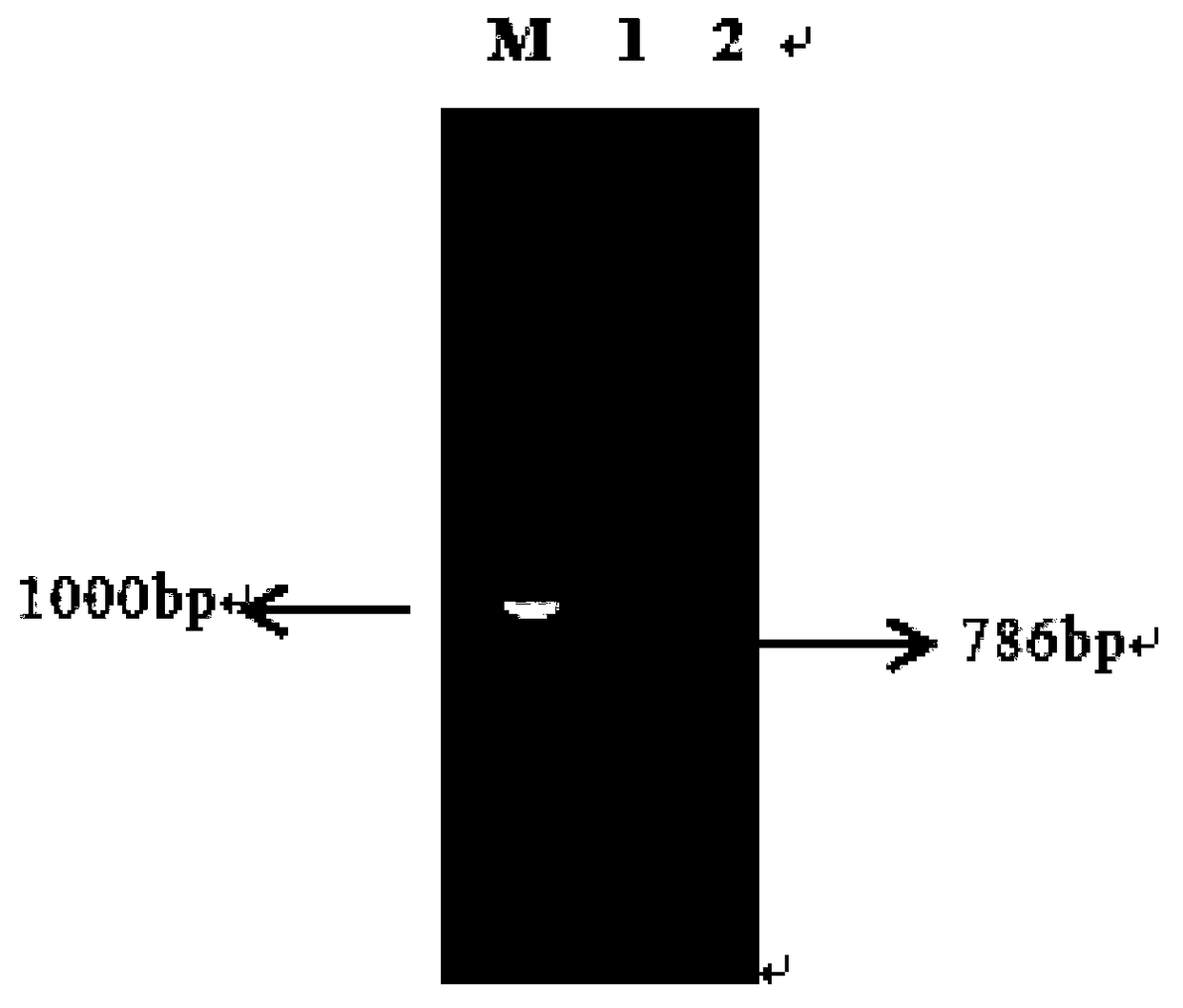
[0050] The GdhBM gene was cloned with primers F_GdhBM / R_GdhBM to obtain a 786bp GdhBM gene (as shown in SEQID NO.2). Nucleic acid electrophoresis to verify gene size, such as figure 2 .
[0051] The sequence of primer F_GdhBM is: 5'-GGA AGATCTG ATGTATAAAGATTTAGAAGGA-3';
[0052] The sequence of primer R_GdhBM is: 5'CCG CTCGAG TTATCCGCGTCCTGCTTGGAA-3'.
[0053] GdhBM gene was digested with glII and XhoI, and the gene band after digestion was recovered. The pET-30a(+) plasmid was digested with BglII and XhoI, and the plasmid band after digestion was recovered. The digested GdhBM gene and enzyme The excised pET-30a(+) plasmid was ligated with ligase and transformed into the cloning host Escherichia coli DH5α. Colony PCR was performed with primers F_GdhBM / R_GdhBM to verify the transformed recombinants, and then the recombinant plasmids were extracted for sequencing. The recombinant plasmid with correct sequencing ...
Embodiment 3
[0054] Construction and induced expression of embodiment 3 genetically engineered bacteria
[0055] The expression host Escherichia coli BL21(DE3) was transformed with the plasmids pET30-LbADH and pET30-GdhBM constructed in Examples 1 and 2, respectively. Colony PCR was performed with primers F_LbADH / R_LbADH and F_GdhBM / R_GdhBM respectively to verify the transformed recombinants. The verified genetically engineered bacteria are EcoLbADH and EcoGdhBM. Inoculate EcoLbADH and EcoGdhBM into 3-5mL liquid LB test tube culture medium containing kanamycin resistance respectively, activate on a shaking table at 35°C for 12 hours, transfer the culture obtained after activation according to 1% transfer amount Into the liquid LB shake flask medium containing kanamycin resistance, culture in the fermentation medium with constant temperature shaking for 3 hours, the culture condition is 37°C, 200rpm. When the cell concentration grows to OD 600 When =0.8, add 0.5mM IPTG (final concentrati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com