PCSK9 iRNA compositions and methods of use thereof
A bonding, AD-60212 technology, applied in PCSK9 iRNA composition and its application field, can solve the problems of myocardial infarction, blood flow occlusion, ruptured clot formation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0676] Example 1. Synthesis of GalNAc-conjugated oligonucleotides
[0677] A series of siRNA duplexes spanning the PCSK9 mRNA sequence were designed and synthesized using the techniques described above and conjugated with trivalent GalNAc at the 3-terminus of the sense strand. The sequences of these duplexes are shown in Table 1. These same sequences were also synthesized with different nucleotide modifications and conjugated with trivalent GalNAc. The sequences of the modified duplexes are shown in Table 2.
[0678]
[0679]
[0680]
[0681]
[0682]
[0683]
[0684]
[0685]
[0686]
[0687]
[0688]
[0689]
[0690]
[0691]
[0692]
[0693]
[0694]
[0695]
[0696]
[0697]
[0698]
[0699]
[0700]
[0701]
[0702]
[0703]
[0704]
[0705]
[0706]
[0707]
[0708]
[0709]
[0710]
[0711]
[0712]
[0713]
[0714]
[0715]
[0716]
[0717] ...
example 2
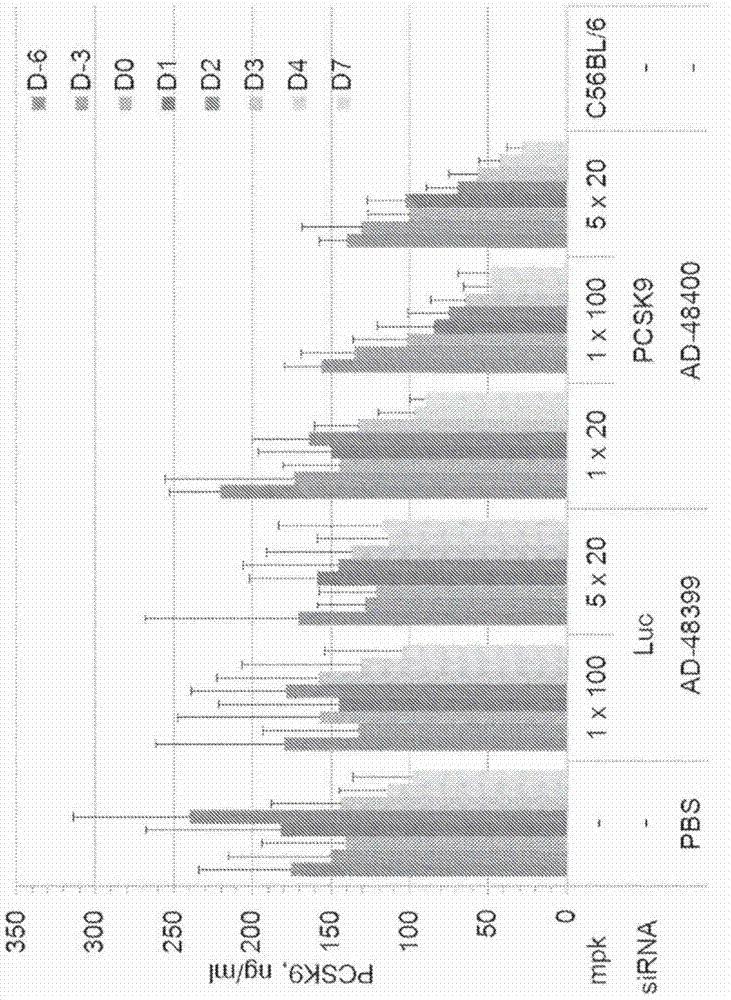
[0763] Example 2. In vitro and in vivo screening.
[0764] The potency of a subset of these duplexes in cynomolgus monkey hepatocytes was assessed in a single-dose ad libitum uptake assay. Briefly, primary cynomolgus monkey hepatocytes (PCH) were treated with conjugated modified siRNA duplexes at three concentrations (500 nM, 100 nM and 10 nM). Free uptake assays at 100 nM and 10 nM were performed in duplicate and data are expressed as average message remaining relative to control + / - standard deviation (SD). Screening at 500 nM was performed in a single pass. Table 3 shows the results of these assays.
[0765] Table 3. PCSK9 potency screen by free uptake in primary cynomolgus monkey hepatocytes
[0766]
[0767]
[0768]
[0769]
[0770]
[0771]
[0772]
[0773] The efficacy of the modified and conjugated PCSK9 siRNA duplexes was also assessed by transfection assays in three human cell lines. PCSK9 siRNA was transfected into three different cell li...
example 3
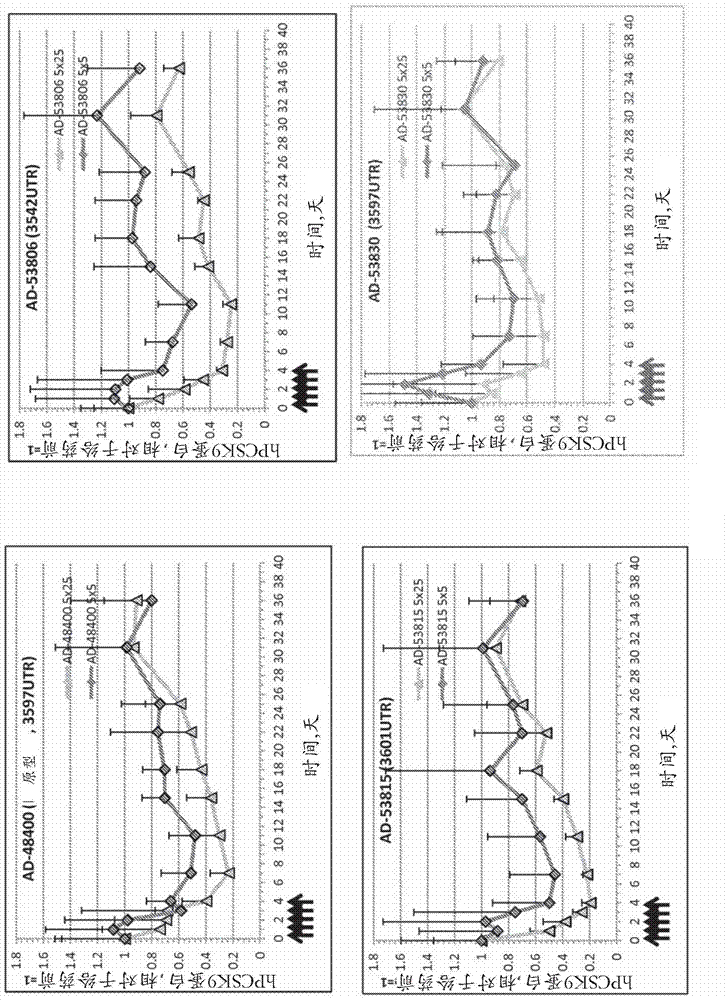
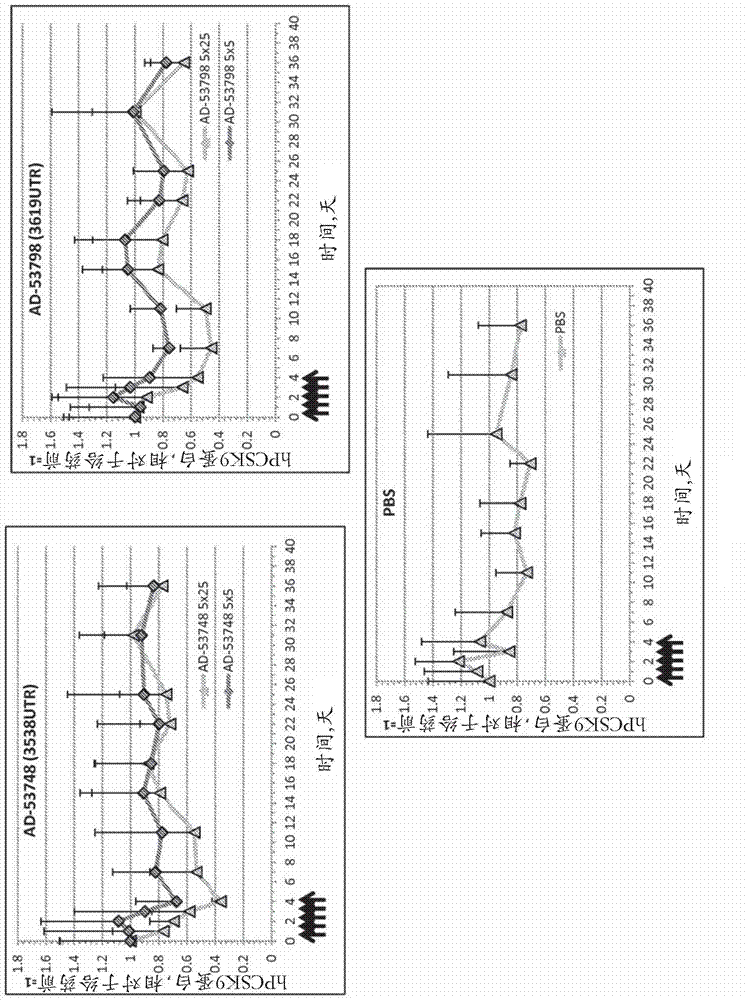
[0788] Example 3. Lead optimization.
[0789] Based on the potency assay described in Example 2 above, PCSK9 with various chemical modifications based on the parental sequences of AD-53815 and AD-53806 was evaluated in primary cynomolgus monkey hepatocytes (PCH) in a free uptake assay Potency of siRNA at 200 nM, 20 nM, 2 nM, and 0.2 nM. For all doses except the 0.2 nM dose, determinations were made in duplicate and data were expressed as the average fraction message remaining relative to control. This 0.2 nM dose was determined as a single shot. The results of these assays are shown in Table 6.
[0790] Table 6. Potency screening for lead optimization of AD-53815 and AD-53806 by free uptake in cynomolgus monkey hepatocytes.
[0791]
[0792]
[0793]
[0794]
[0795]
[0796]
[0797]
[0798] siRNAs based on the parental sequences of AD-53815 and AD-53806 with various chemical modifications were also screened for in vitro potency by transfection in He...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com