Pyrazolone compound and application
A technology of pyrazolones and compounds, applied in the field of biomedical applications of pyrazolone compounds and their applications, potent inhibitors, which can solve the problems of affecting bioavailability, not relieving delayed diarrhea, reducing drug dosage, etc. problems, achieve high inhibitory activity, simple synthesis process, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
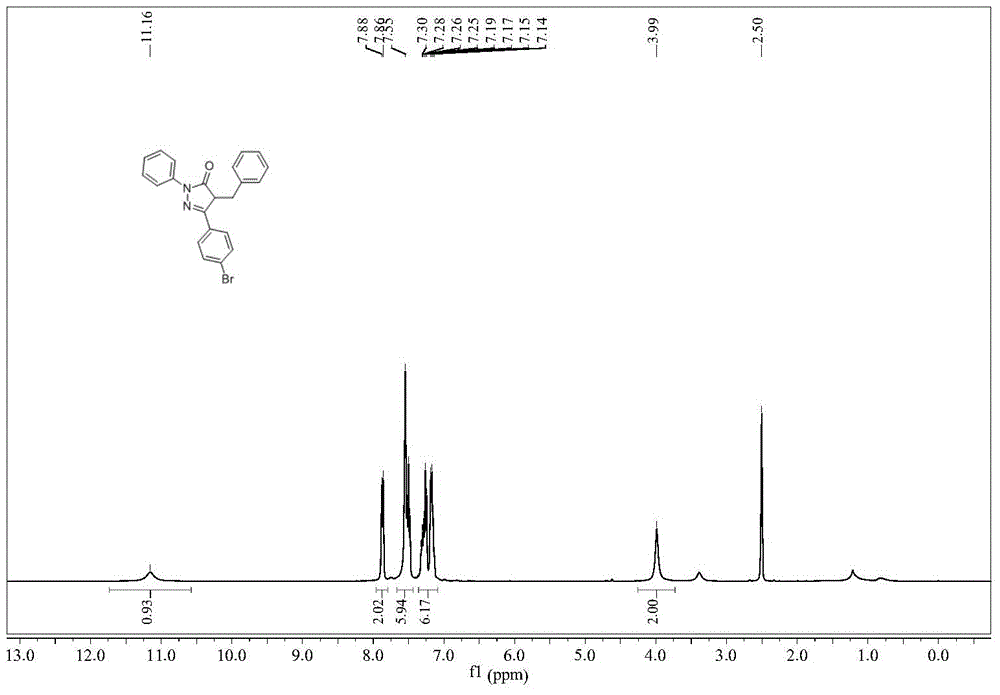
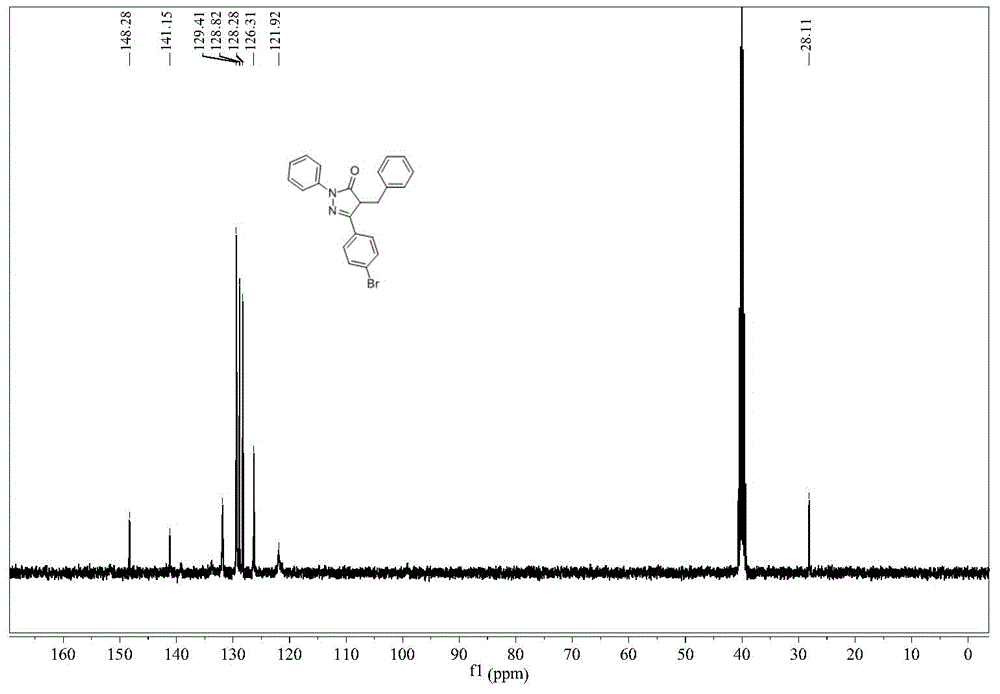
[0029] Example 1 Synthesis of 1-phenyl-3-(4-bromobenzyl)-4-benzyl-5-pyrazolone
[0030] The synthetic route of 1-phenyl-3-(4-bromobenzyl)-4-benzyl-5-pyrazolone is:
[0031]
[0032] Specifically through the following methods:
[0033] 1) Synthesis of compound 1 (methyl p-bromobenzoylacetate)
[0034]At room temperature, NaH (8.4g, 210mmol), dimethyl carbonate (17.6mL, 210mmol), and 30mL toluene were added to a 250mL three-necked flask, the temperature was raised to 120°C and refluxed, and p-bromoacetophenone (16.5g, 83mmol) in 30mL toluene solution. The reaction was monitored by thin-plate chromatography (TLC). After 30 minutes, the reaction of benzene-to-bromoethanone was complete, cooled to room temperature, added 100 mL of ice water, and 6N HCl solution to adjust the pH to 6-7. Separate the organic phase, extract the aqueous phase with ethyl acetate three times (100 mL×1, 50 mL×2), combine the organic phases, wash once with water (50 mL×1), wash once with saturated b...
Embodiment 2
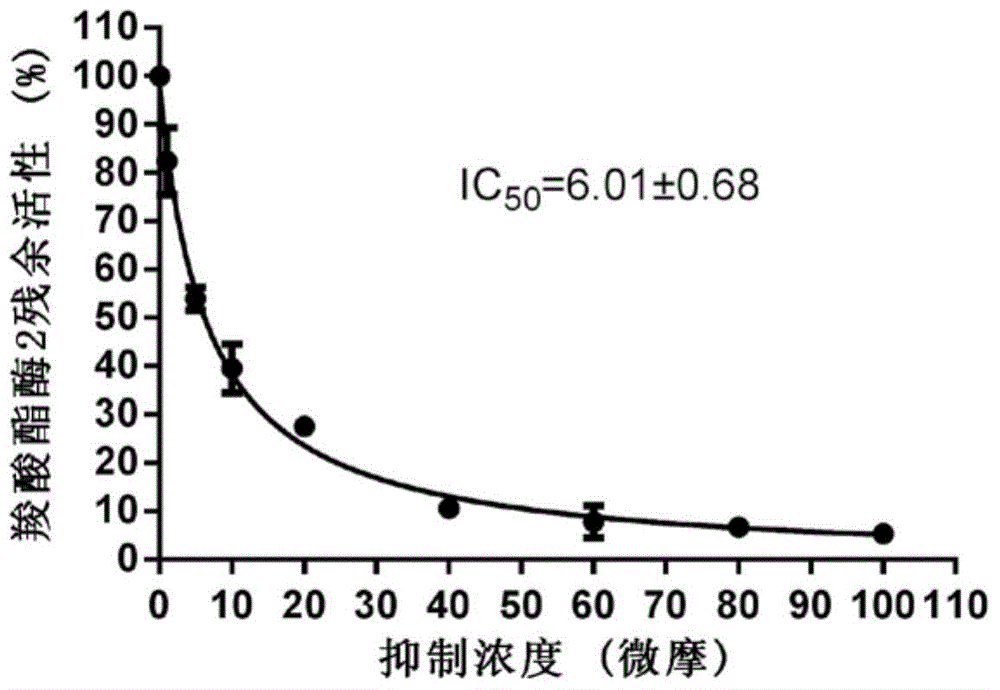
[0041] Example 2 Quantitative evaluation of formula I pyrazolone compounds on carboxylesterase 2 inhibitory ability
[0042] Using the hydrolytic metabolism of fluorescein diacetate as a probe reaction, the IC of inhibition of carboxylesterase 2 by pyrazolone compounds was determined by means of human liver microsome in vitro incubation system 50 :
[0043] 1) In 200 microliters of in vitro metabolic reaction system, containing phosphate buffer solution with a pH of 7.4, the concentration of human liver microsomal protein is 2 μg / ml, and the final concentration of inhibitors is in the range of 0.01 μM-100 μM. Shake pre-incubation at 37°C 10 minutes;
[0044] 2) Add the substrate (final concentration 10 μM) to the reaction system to initiate the reaction; after reacting at 37°C for 30 minutes, add 200 μl of acetonitrile, shake vigorously, and terminate the reaction;
[0045] 3) Using a high-speed refrigerated centrifuge, under the condition of 20,000×g, centrifuge the above s...
Embodiment 3
[0051] Example 3 1-Phenyl-3-(4-bromobenzyl)-4-benzyl-5-pyrazolone on slowing down irinotecan-induced diarrhea in mice
[0052] Twenty-one Balb / c mice were randomly divided into 3 groups: normal control group, irinotecan diarrhea model group and 1-phenyl-3-(4-bromobenzyl)-4-benzyl-5-pyrazoline Ketone + irinotecan diarrhea model group (referred to as pyrazolone group), 7 rats in each group. The diarrhea status of the mice in the normal control group, the diarrhea model group and the pyrazolone group were observed respectively, and the intestinal tissues of the mice were examined by histological section. The diarrhea model group followed the Trifan method (Cancer Res 2002; 62:5778-84.), and the diarrhea model was injected with irinotecan (100mg / kg / d) for 3 consecutive days (d). It appeared every day, and it was the most serious on the 4th day. Pyrazolone was started 3 days before CPT-11 injection, once a day, 100 mg / kg was administered by intragastric administration, and the ot...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap