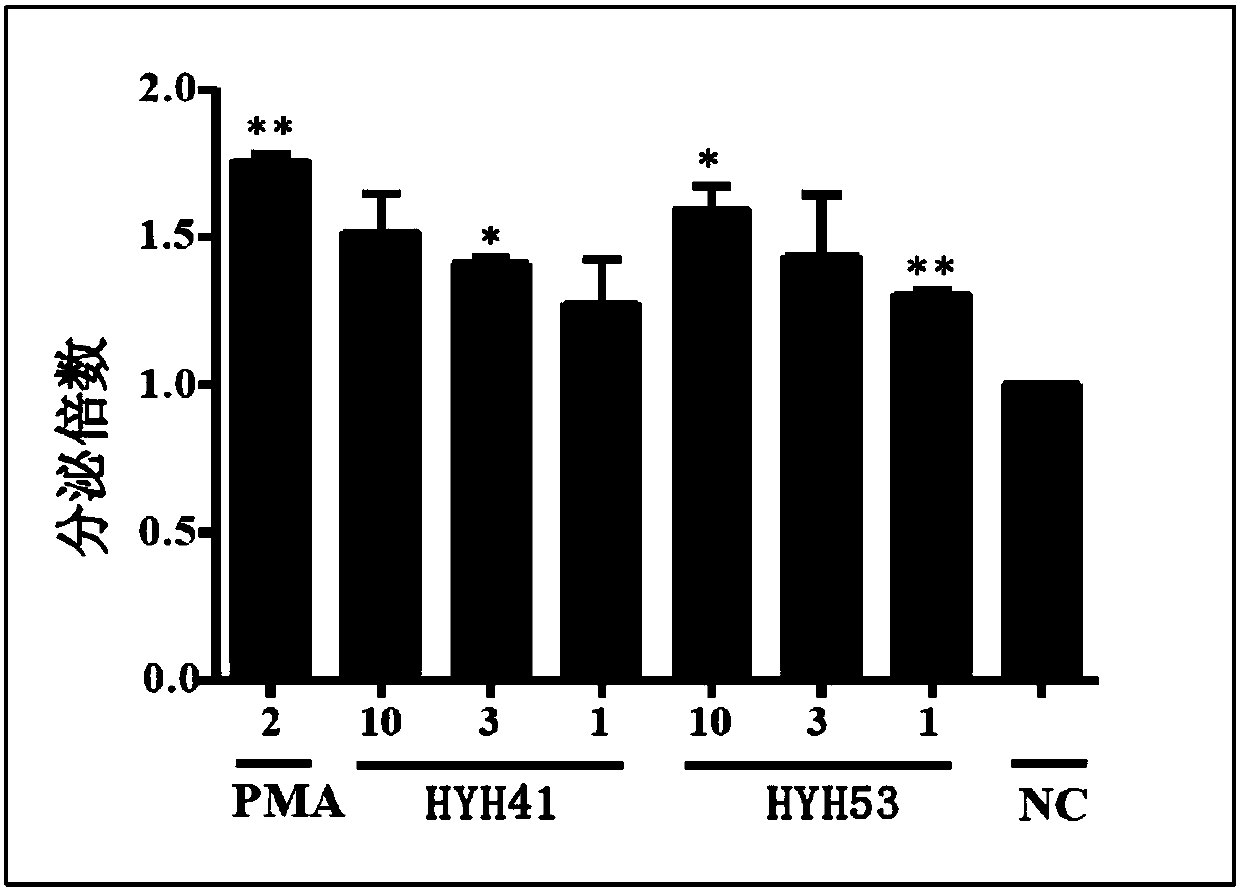
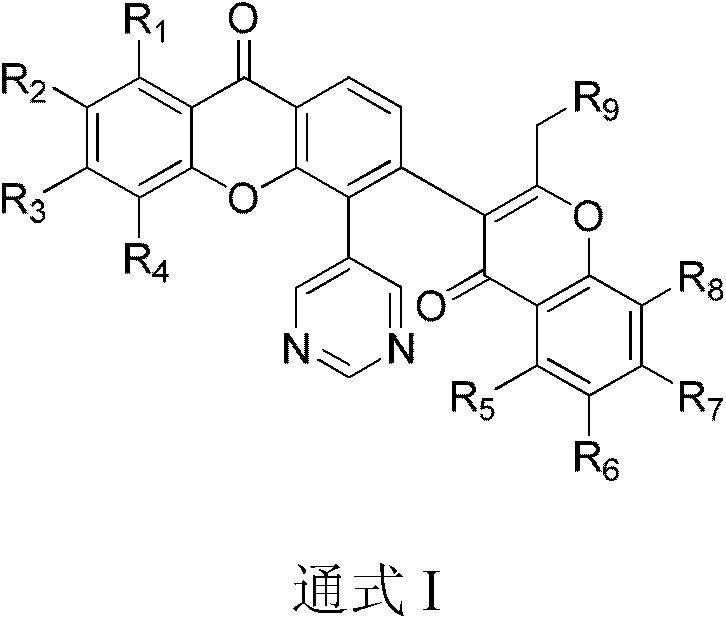
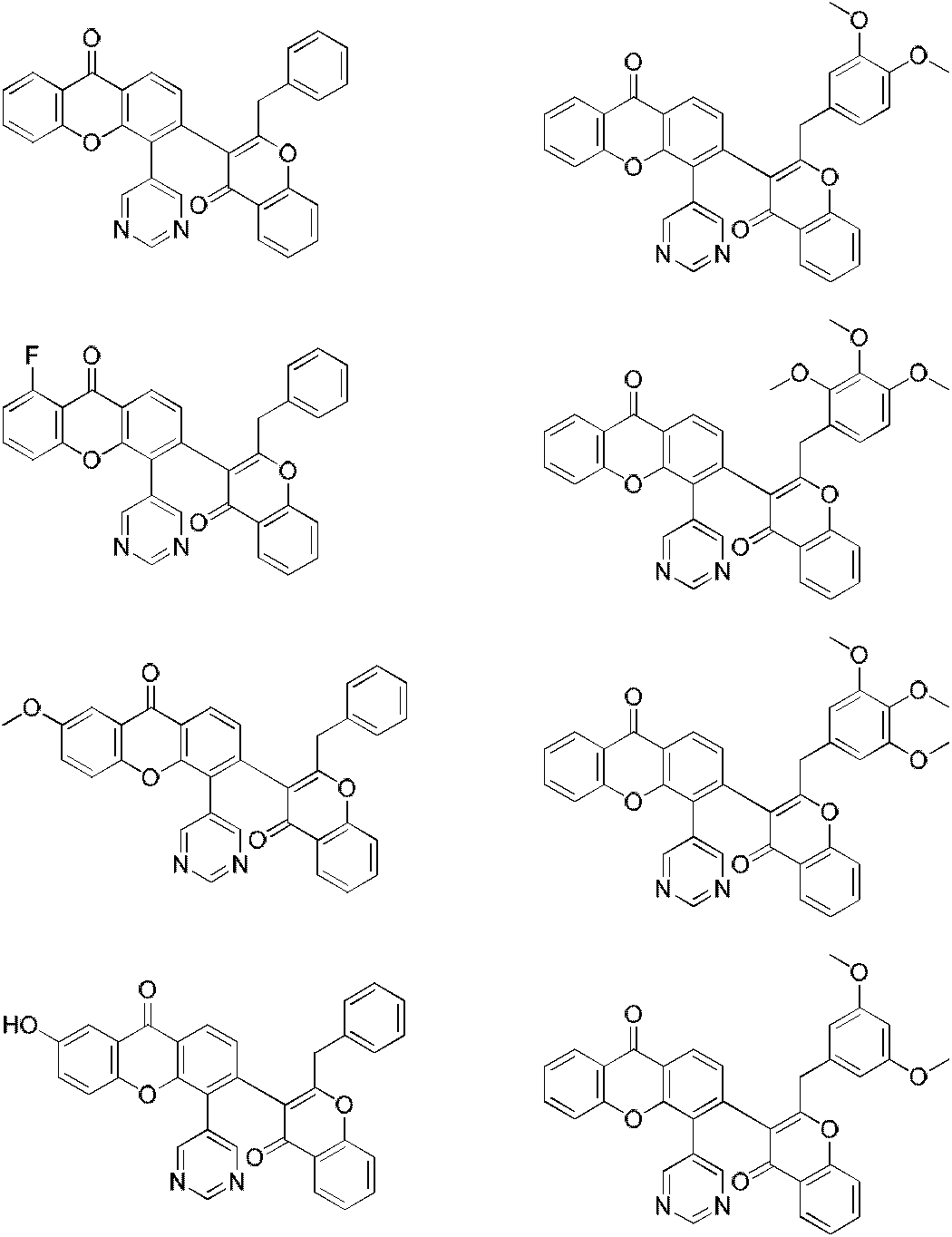
A class of xanthochromone compounds and their preparation method and use
A technology of xanthochromone and compounds, applied in the field of pharmacy, can solve problems such as gallbladder toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0064] The preparation of embodiment 1 compound HYH1
[0065]
[0066] Step a: o-hydroxyacetophenone (compound 1, 10g, 73.5mmol, 1eq) was dissolved in N,N-dimethylformamide dimethyl acetal (DMF-DMA) 15ml, 113.9mmol, 1.55eq), The mixture was reacted at 100°C for 2 hours, and the reaction was completed. Cooling, precipitated solid, filtered, and washed with 30ml ethyl acetate to obtain intermediate product (E)-3-(dimethylamino)-1-(2-hydroxyphenyl)-propenone (compound 2), 1 H NMR (300MHz, CDCl 3 )δ9.20(s, 1H), 8.08(s, 1H), 7.51(m, 2H), 7.03(s, 1H), 4.60(s, 1H), 2.91(s, 6H).
[0067] Step b: (E)-3-(dimethylamino)-1-(2-hydroxyphenyl)-propenone (13g, 68mmol, 1eq) and elemental iodine (34.5g, 136mmol, 2eq) and pyridine (5.5ml , 68mmol, 1eq) was dissolved in 150ml chloroform and reacted for 6 hours, and the reaction was completed. Cool, add 150ml sodium sulfite saturated solution to remove excess iodine, extract three times with 150ml ethyl acetate respectively, combine organic...
Embodiment 2
[0076] The preparation of embodiment 2 compound HYH2
[0077] It was prepared in the same manner as in Example 1 except that 6-fluoro-o-hydroxyacetophenone was used instead of o-hydroxyacetophenone.
[0078]
[0079] 1 H NMR (300MHz, CDCl 3 )δ9.10(s,1H),8.57–8.44(m,2H),7.72–7.54(m,3H),7.42(d,J=3.0Hz,1H),7.36(dd,J=8.7,5.2Hz ,2H),7.30–7.20(m,4H),7.15–6.94(m,4H),3.83(s,2H).
Embodiment 3
[0080] The preparation of embodiment 3 compound HYH3
[0081] It was prepared in the same manner as in Example 1 except that 5-methoxy-o-hydroxyacetophenone was used instead of o-hydroxyacetophenone.
[0082]
[0083] 1 H NMR (300MHz, CDCl 3 )δ9.10(s,1H),8.53(d,J=10.2Hz,3H),7.73(s,1H),7.44(s,1H),7.39–7.31(m,2H),7.24(d,J =16.2Hz,7H),7.02(s,2H),3.93(s,3H),3.81(s,2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com