Polypeptide compound and application thereof
A technology of peptide compounds and stereoisomers, applied in the field of peptide compounds and their applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~54
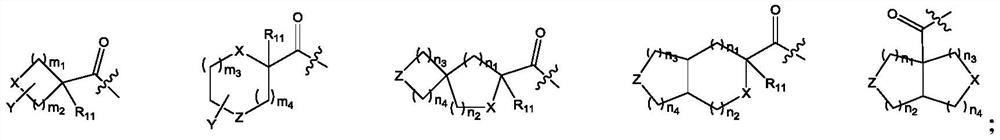
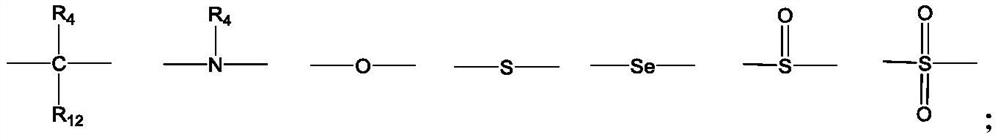
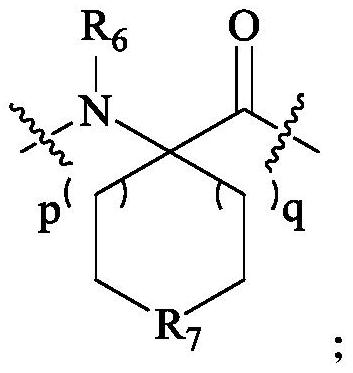
[0138] Preparation of compounds 1-54
[0139] Polypeptide synthesis adopts standard Fmoc solid-phase method, selects Rink Amide resin, binds the C-terminal amino acid to the resin, and extends the peptide chain from the C-terminal to the N-terminal. The protected amino acids used in the present invention include: Fmoc-Apc(Boc)-OH, Fmoc-D-Lys(Boc)-OH, Fmoc-D-Orn(Boc)-OH, Fmoc-Phe-OH, Fmoc-D-Trp (Boc)-OH, Fmoc-D-Bal-OH, Fmoc-D-Cit-OH, Fmoc-D-Arg(Pbf)-OH, Boc-D-Aba-OH, Fmoc-Lys(Boc)-OH, Fmoc-Lys(Alloc)-OH, Fmoc-Gly-OH, Fmoc-D-Ala-OH, Fmoc-D-Val-OH, Fmoc-D-Leu-OH, Fmoc-Pro-OH, Fmoc-β-Ala -OH, Fmoc-D-Tle-OH, Fmoc-Tle-OH, Fmoc-D-Nle-OH, Fmoc-Nle-OH, cis-2-(tert-butoxycarboxamide)-1-cyclopentanecarboxylic acid , Boc-methyl-1-(aminomethyl)cyclobutanecarboxylic acid, 1-N-Boc-3-azetidinecarboxylic acid, Boc-3-aminooxetane-3-carboxylic acid, 1- Boc-D-acridine-2-carboxylic acid, (S)-1-Boc-pyrrolidine-3-carboxylic acid, Boc-2-morpholinecarboxylic acid, (Boc-3-amino-1-adamantane)acetic a...
Embodiment 55
[0161] The agonistic activity evaluation experiment of polypeptide compounds on GHSR-1a (EC 50 )
[0162] Screening of GHSR-active compounds is accomplished by recombinantly expressing the receptor. The use of recombinant expression of GHSR offers several advantages, such as the ability to express the receptor in a defined cellular system so that the response of a compound to GHSR can be more easily distinguished from that of other receptors. For example, GHSR can be expressed in cell lines such as HEK293, COS7, and CHO that normally express GHSR without an expression vector, and the same cell line without an expression vector can be used as a control.
[0163] The activity of GHSR-1a can be measured using different techniques, eg, by detecting changes in the intracellular conformation of GHSR-1a, changes in G-protein coupling activity, and / or changes in intracellular messengers, among others. Preferably, methods such as measuring intracellular Ca 2+ technique to measure GH...
Embodiment 56
[0193] Inhibition of Cytochrome P450 Oxidase (CYP450) by Polypeptide Compounds
[0194] The metabolism of drugs in vivo can be divided into phase I reactions (catabolism) and phase II reactions (anabolism). Phase I reactions include oxidation, reduction and hydrolysis reactions, mainly catalyzed by cytochrome P450 (CYP450) enzymes. CYP450 enzymes are mainly distributed in the liver, so they are also called liver drug enzymes. Induction and inhibition of CYP450 enzymes have important clinical implications and can lead to clinical drug interactions. Therefore, the inhibition experiment of CYP450 is often used as a safety index to evaluate the druggability of drugs.
[0195] Compounds 1 to 44 obtained in Examples 1 to 44 were used as test compounds. Human liver microsomes containing cytochrome P450 (0.253 mg / mL protein) were combined with test compounds (0.05 to 50 μM), CYPs substrate (10 μM p-acetamido) phenol, 5 μM diclofenac, 30 μM mephentoin, 5 μM dextromethorphan hydrobro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com