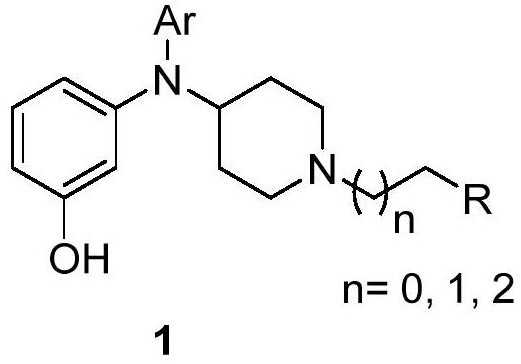
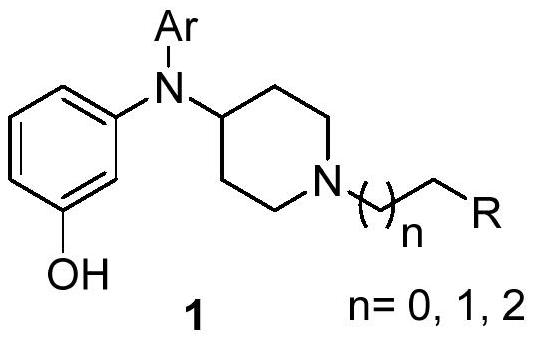
N, N-(4-piperidyl, aryl)-3-aminophenol derivative, pharmaceutical composition and application thereof
A technology based on aminophenol and piperidinium, which is applied in the field of medicine, can solve the problems of unseen and no pharmacological activity, and achieve the effects of simple and easy preparation method, high triple agonist activity, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
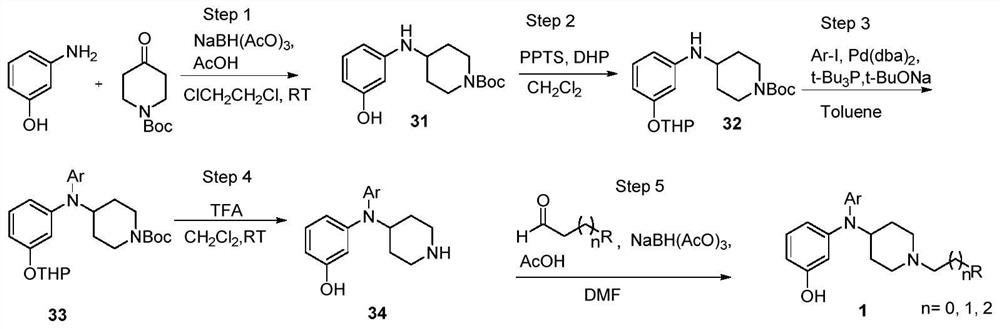
[0048] Synthetic general formula:
[0049]
[0050] Synthesis of intermediate 31:
[0051] Add 3-aminophenol (5.0g, 45.9mmol, 1.0eq) dissolved in 1,2-dichloroethane (150mL) under stirring at room temperature into a reaction flask treated with anhydrous and oxygen-free treatment, and then add N-tert-butoxy Carbonyl-4-piperidone (9.2 g, 45.8 mmol, 1.0 eq), sodium triacetoxyborohydride (14.6 g, 68.7 mmol, 1.5 eq) and glacial acetic acid (4.1 mL, 68.7 mmol, 1.5 eq). After 24h, it was quenched by adding 1N sodium hydroxide solution (76.1mL, 76.1mmol). The post-experiment treatment was performed according to the general experimental procedure, and the intermediate 31 (9.9g, 74.7%) was obtained by column chromatography purification.
[0052] Synthesis of Intermediate 32:
[0053] Add compound 31 (5.0 g, 17.1 mmol, 1.0 eq) dissolved in ultra-dry dichloromethane (35 mL) under stirring at room temperature into a pressure bottle treated with anhydrous and oxygen-free treatment, and th...
Embodiment 2
[0091] biological activity. Evaluation of the agonist activity of the compounds of the invention on opioid receptors (MOR, DOR, KOR).
[0092] In order to evaluate the agonistic effect of the compounds of the present invention on opioid receptors (MOR, DOR, KOR), the present invention uses drugs DAGO (MOR agonist), DPDPE (DOR agonist), U50488 (KOR agonist) as positive controls. Compound EC 50 Values were determined by concentration-effect curve generation calculations.
[0093] 1. Experimental principles and methods
[0094] Experimental principle: By establishing a cell line that co-transforms the target receptor and Gα16, the activation of the receptor can cause the activation of Gα16 protein, and then activate phospholipase C (PLC) to produce IP3 and DAG, and IP3 can bind to the endoplasm in the cell Reticulum binds to the IP3 receptor on the mitochondria, thereby causing the release of intracellular calcium. Therefore, measuring the change of intracellular calcium ca...
Embodiment 3
[0102] Preparation of tablets:
[0103] Take N,N-(4-piperidinyl, aryl)-3-aminophenol derivatives (any one or any combination of compounds 2-30) and their polymorphs, according to their weight ratio with excipients Add excipients at a ratio of 1:5-1:10, granulate and compress into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com