Endomorphin analog modified by hydroxylation of 4-phenylpropanamide and its synthesis method and application
A technology of phenylpropanamide hydroxyl and endomorphin, applied in the direction of drug combination, tetrapeptide composition, peptide/protein composition, etc., can solve blood pressure reduction, cardiovascular system side effects, affinity, opioid agonist activity, and analgesia Features need to be improved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
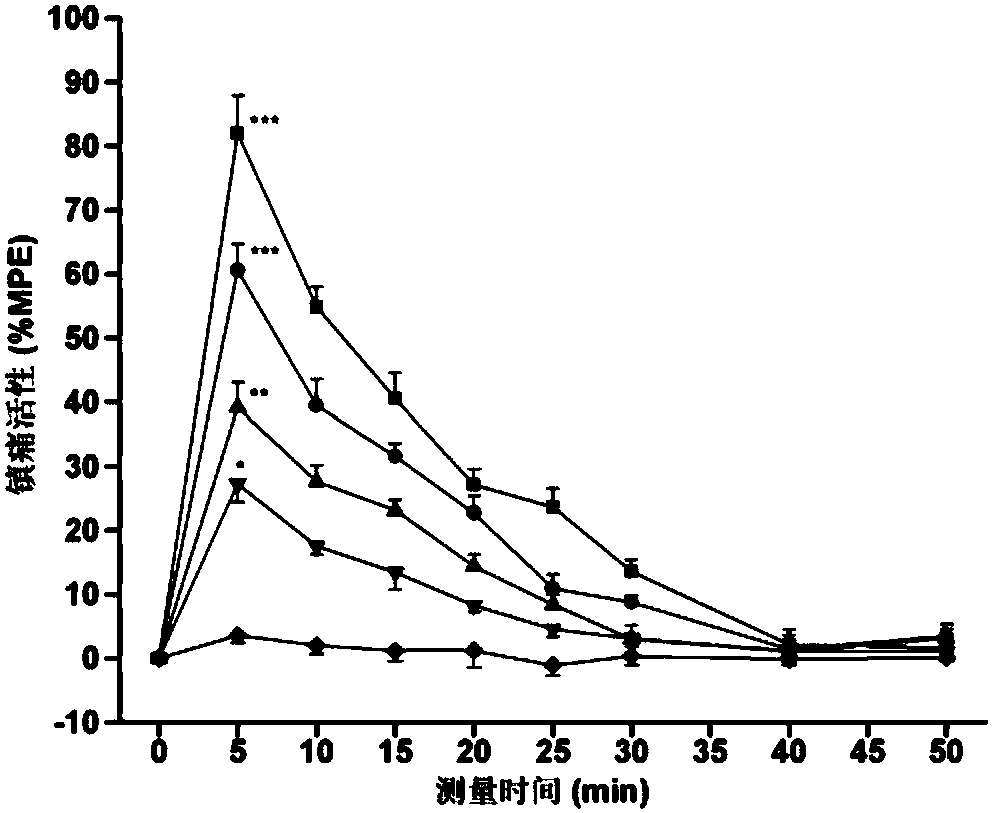
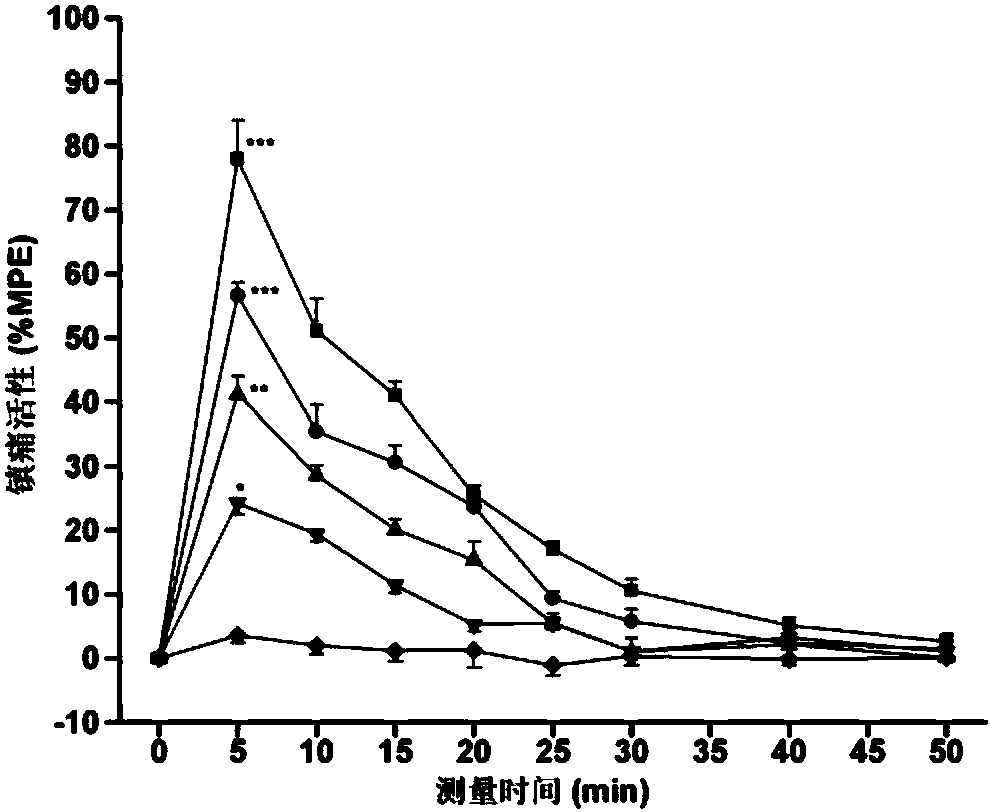
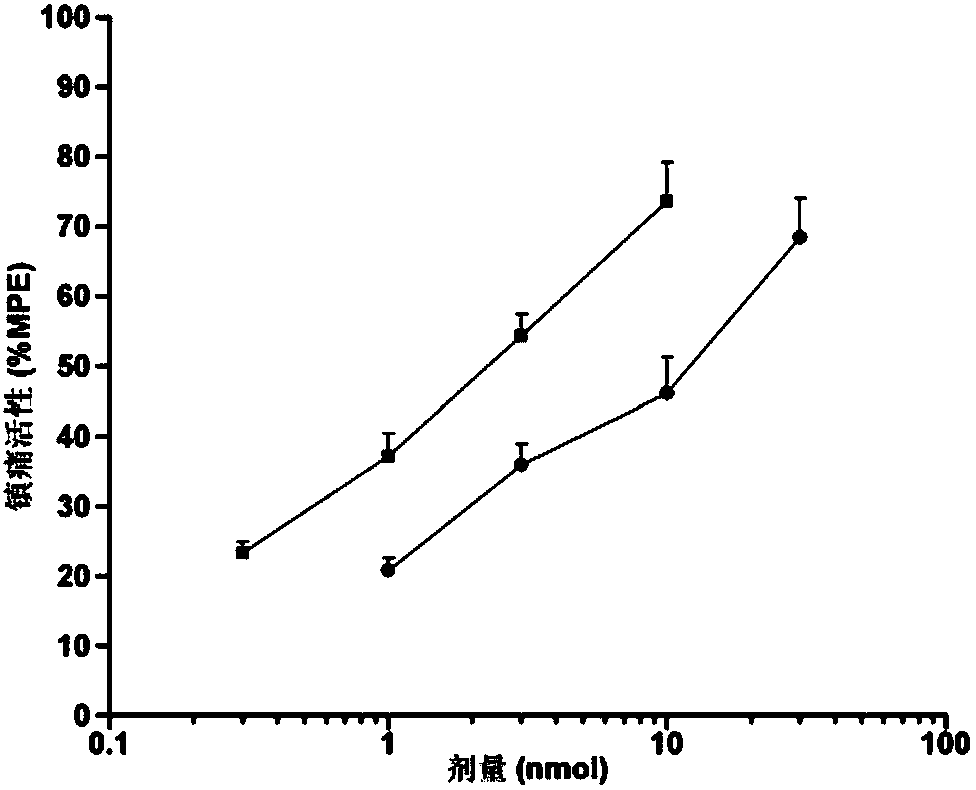
[0076] Specific embodiment 1: The endomorphin analogue modified by the hydroxylation of the 4-phenylpropanamide in this embodiment is an endomorphin-1 analogue, tyrosine-proline-tryptophan-phenylpropanoid (N-hydroxyl) Amide, its structure is as follows:
[0077]
specific Embodiment approach 2
[0078] Specific embodiment two: the synthesis method of endomorphin analogs modified by hydroxylation of 4-phenylpropanamide in the present embodiment comprises the following steps:
[0079] One, the synthesis of N-tert-butoxycarbonyl-phenylpropanoid (N-hydroxyl) amide
[0080] Add N-tert-butoxycarbonyl-phenylalanine and N-hydroxysuccinimide into redistilled tetrahydrofuran, stir at 0-5°C to obtain reaction solution A; wherein N-tert-butoxycarbonyl-phenylalanine The molar ratio of N-hydroxysuccinimide is 1:1.2~1.4;
[0081] Dissolve N,N'-dicyclohexylcarbodiimide in tetrahydrofuran, add it to reaction solution A, stir and react at 0-5°C for 20-40 minutes, and then react at room temperature for 4-8 hours; where N-tert-butoxycarbonyl- The molar ratio of phenylalanine to N,N'-dicyclohexylcarbodiimide is 1:1.2~1.4;
[0082] Filter out the product 1,3-dicyclohexyl urea, retain the activated ester filtrate, dissolve hydroxylamine hydrochloride in water, adjust the pH value to 9-11,...
specific Embodiment approach 3
[0103] Specific embodiment three: the difference between this embodiment and specific embodiment two is: the washing method in step one is: wash with 5% citric acid solution, saturated sodium bicarbonate solution and saturated sodium chloride solution successively. Others are the same as in the second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com