KCNQ potassium ion channel agonist, pharmaceutical composition and application of KCNQ potassium ion channel agonist
A potassium ion channel and agonist technology, applied in the field of pharmacy, can solve the problems of low agonistic activity, insufficient selectivity, and reduced electron cloud density, and achieve the effect of high agonistic activity and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
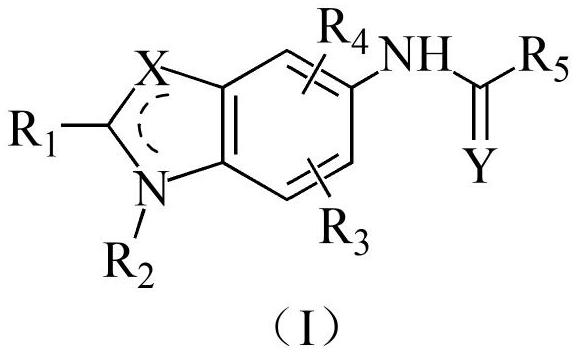
[0060] Embodiment 1: It is a KCNQ potassium ion channel agonist disclosed by the present invention, a compound having a structure shown in the following formula (I) or a pharmaceutically acceptable salt thereof,
[0061]
[0062] Among them, R 1 selected from H, halogen, substituted or unsubstituted phenyl, or substituted or unsubstituted phenylalkyl, the substituents of phenyl and phenylalkyl are each independently selected from halogen or haloalkyl;
[0063] X is selected from S or C;
[0064] R 2 optionally selected from H, alkyl, alkenyl or alkynyl;
[0065] R 3 and R 4 each independently selected from H or alkyl;
[0066] Y is selected from O or S;
[0067] R 5 selected from substituted or unsubstituted alkyl, substituted or unsubstituted cycloalkyl, alkoxy or furyl, the substituent of alkyl selected from alkoxy, dialkylamino or alkoxycarbonyl, cycloalkyl The substituents for are selected from halogen.
Embodiment 2
[0068] Embodiment 2: a KCNQ potassium ion channel agonist disclosed by the present invention, the difference from Example 1 is that R 2 Choose from H, C 1 ~C 3 Alkyl, C 1 ~C 3 Alkenyl or C 1 ~C 3 Alkynyl.
[0069] R 3 and R 4 each independently selected from H or C 1 ~C 6 alkyl.
[0070] R 5 selected from substituted or unsubstituted C 1 ~C 6 Alkyl, substituted or unsubstituted C 3 ~C 6 Cycloalkyl, C 1 ~C 6 Alkoxy or furyl, the substituent of alkyl is selected from C 1 ~C 6 Alkoxy, two (C 1 ~C 4 Alkyl) amino or C 1 ~C 6 Alkoxycarbonyl, cycloalkyl substituents are selected from halogen.
Embodiment 3
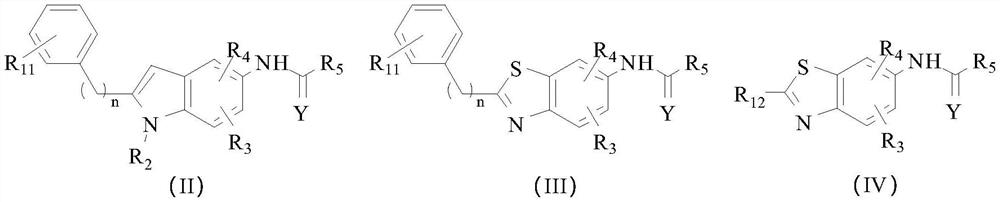
[0071] Embodiment 3: It is a KCNQ potassium ion channel agonist disclosed by the present invention. The difference from Example 1 is that the compound has a structure selected from the following formulas (II) to (IV),
[0072]
[0073] Among them, n≥0;
[0074] R 11 and R 12 each independently selected from H, halogen or halomethyl;
[0075] R 2 Choose from H, C 1 ~C 3 Alkyl, C 2 ~C 3 Alkenyl or C 2 ~C 3 Alkynyl;
[0076] R 3 and R 4 each independently selected from H or C 1 ~C 6 alkyl;
[0077] Y is selected from O or S;
[0078] R 5 selected from substituted or unsubstituted C 1 ~C 6 Alkyl, substituted or unsubstituted C 3 ~C 6 Cycloalkyl, C 1 ~C 6 Alkoxy or furyl, the substituent of alkyl is selected from C 1 ~C 6 Alkoxy, two (C 1 ~C 4 Alkyl) amino or C 1 ~C 6 Alkoxycarbonyl, cycloalkyl substituents are selected from halogen.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com