Preparation method of disubstituted benzothiophene potassium ion channel agonist
A potassium ion channel, benzothiophene technology, used in organic chemistry, nervous system diseases, drug combinations, etc., can solve the problems of compounds without reducing electron cloud density, side effects, and easy oxidation of intermediate aromatic rings.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
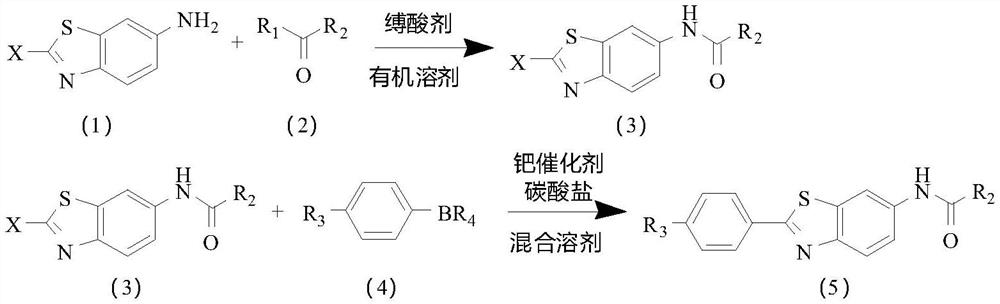
[0041] Embodiment 1: It is a preparation method of a disubstituted benzothiophene potassium ion channel agonist disclosed in the present invention. The route of the preparation method is as follows,
[0042]
[0043] The preparation method comprises the following steps,
[0044] S1 puts the compound of the above formula (1) and the compound of the above formula (2) in an organic solvent, selectively adds a condensing agent, and then reacts in the presence of an acid-binding agent, and after the reaction is finished, the above formula (3) is obtained by post-processing compound;
[0045] S2 In a mixed solvent, under the action of a palladium catalyst and a carbonate, the compound of the above formula (3) reacts with the compound of the above formula (4), and after the reaction is finished, the compound of the above formula (5) is obtained through post-treatment;
[0046] Wherein, X is selected from halogen;
[0047] R 1 selected from halogen, hydroxy or alkoxy;
[0048] ...
Embodiment 2
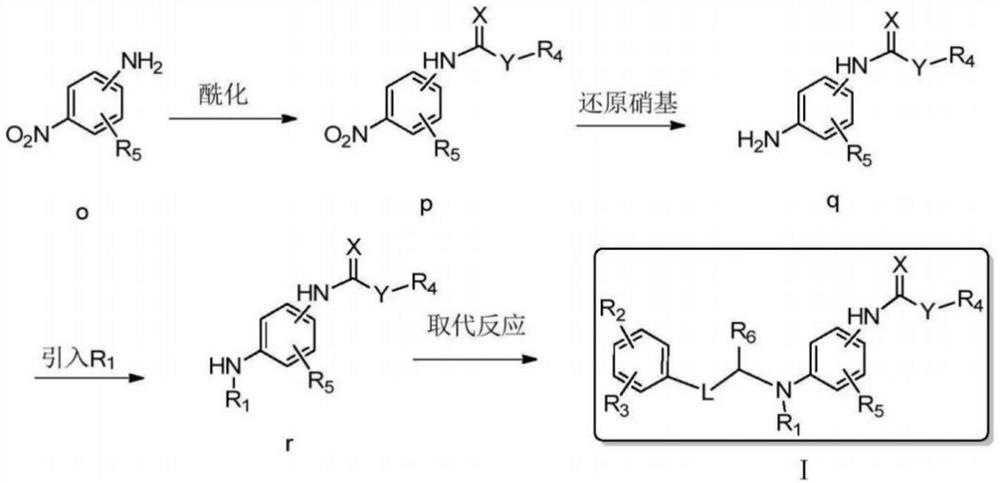
[0051] Embodiment 2: It is a preparation method of a disubstituted benzothiophene potassium ion channel agonist disclosed in the present invention. The difference from Example 1 is that the route of the preparation method is as follows,
[0052]
[0053] The preparation method comprises the following steps,
[0054] S1 Dissolve 1.0mol of the above formula (1) compound, 1.1mol of the above formula (2) compound and 1.2mol of carbodiimide (condensing agent) in 15L of dichloromethane (organic solvent), add 1.5mol of N,N- Diisopropylethylamine (acid-binding agent), stirred and reacted at 23°C for 4h;
[0055] Thin-layer chromatography shows that after the reaction of the raw materials is complete, first slowly add 50L of ethyl acetate to the reaction solution for extraction, stir and separate layers to obtain the first organic phase, then add 25L of ethyl acetate to the separated aqueous phase, and stir Separate the layers to obtain the second organic phase, combine the first o...
Embodiment 3
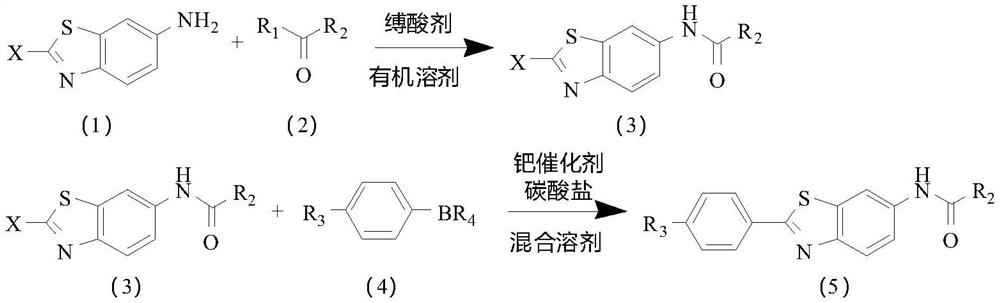
[0060] Example 3: a method for preparing a disubstituted benzothiophene potassium ion channel agonist disclosed in the present invention, the difference from Example 1 is that the route of the preparation method is as follows,
[0061]
[0062] The preparation method comprises the following steps,
[0063] S1 Dissolve 1.0mol of the above formula (1) compound, 1.6mol of the above formula (2) compound and 0.8mol of carbodiimide (condensing agent) in 15L of dichloromethane (organic solvent), add 1.2mol of N,N- Diisopropylethylamine (acid-binding agent), stirred and reacted at 20°C for 6h;
[0064] Thin-layer chromatography shows that after the reaction of the raw materials is complete, first slowly add 50L of ethyl acetate to the reaction solution for extraction, stir and separate layers to obtain the first organic phase, then add 25L of ethyl acetate to the separated aqueous phase, and stir Separate the layers to obtain the second organic phase, combine the first organic pha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com