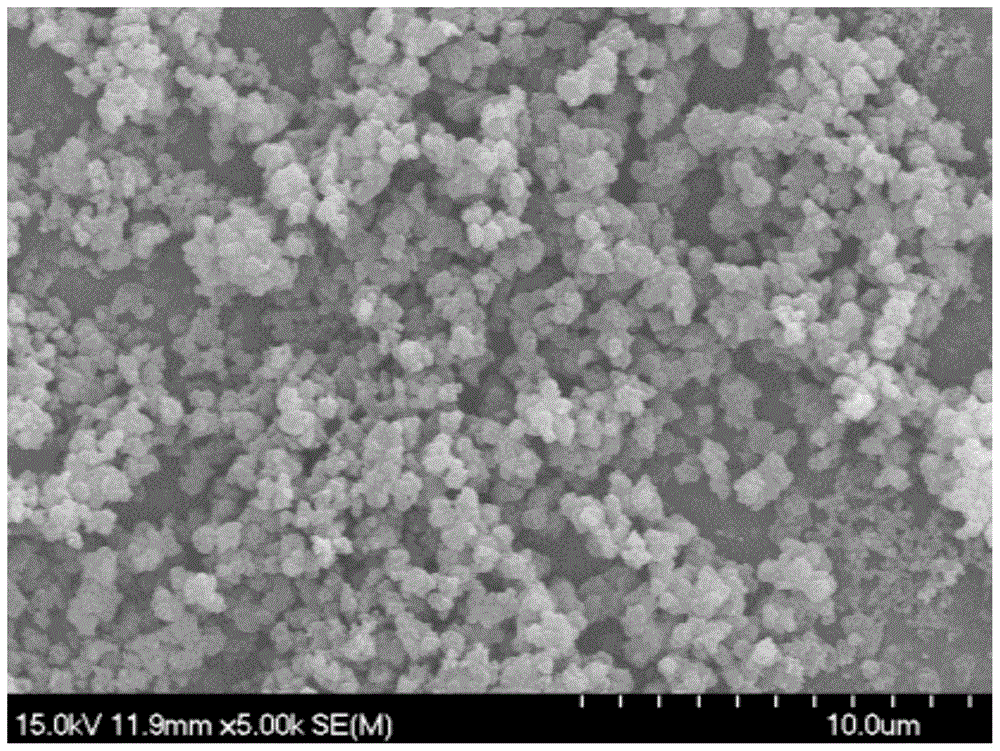
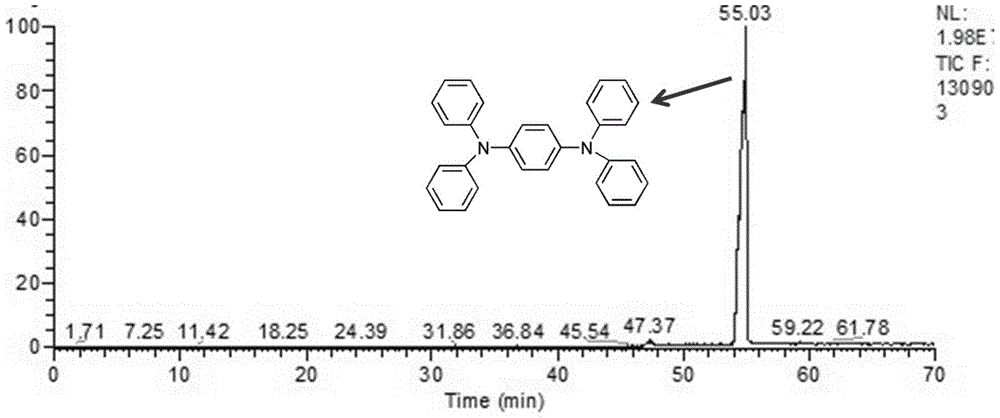
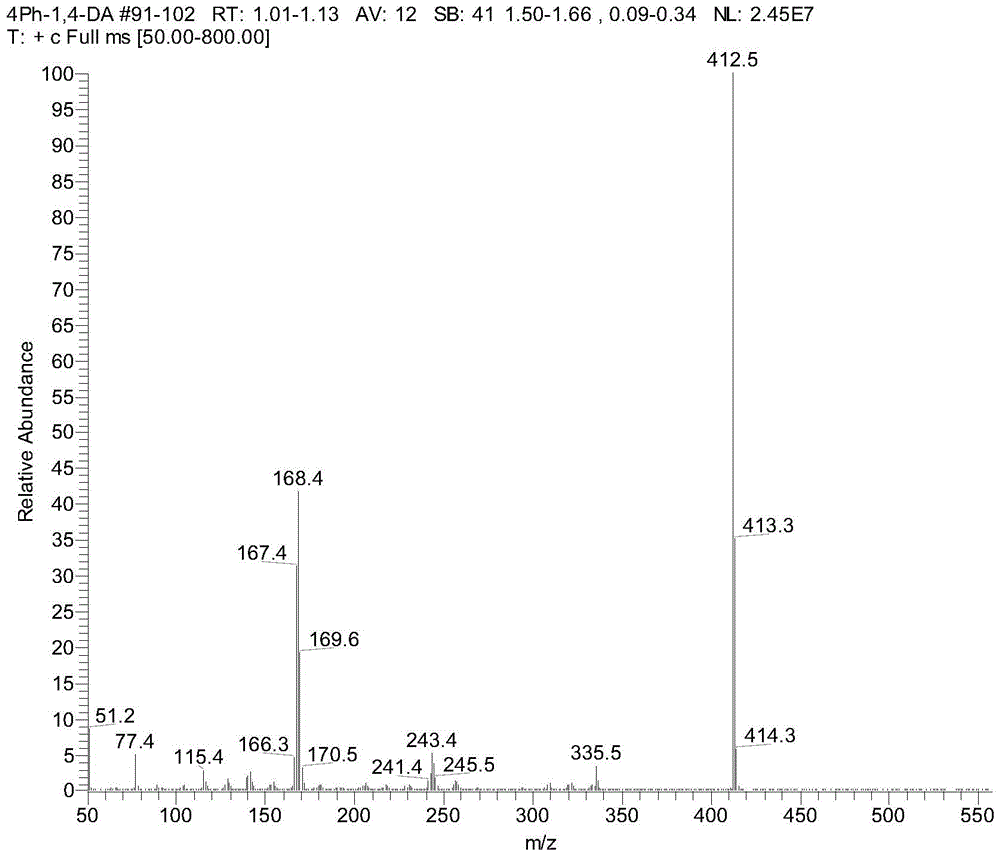
Polydiphenyltriphenylamine, its application and lithium-ion battery made therefrom
A technology of lithium-ion batteries and terphenylamine, which is applied in the direction of battery electrodes, circuits, electrical components, etc., can solve the problem of low discharge specific capacity, achieve increased free radical density, high charge-discharge specific capacity, and improve electron transport performance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: polydiphenylamine
[0036] Synthesis of triphenylamine: add 3.5g (20mmol) diphenylamine, 2.36g (10mmol) 1,4-dibromobenzene, 1.5g sodium tert-butoxide, 0.1g palladium acetate to a pre-dried 250mL three-necked flask , then add 40mL of toluene solution, stir evenly, inject 3mL tri-tertbutylphosphine into the reaction system with a syringe under the protection of nitrogen, and reflux at 110°C for 12 hours. After the reaction, the extraction reaction solution was repeatedly washed with dichloromethane and saturated brine for several times to obtain an extract, and then the crude product was extracted by thin-layer chromatography. 300-mesh silica gel was used as the stationary phase, and n-hexane / ethyl acetate was used as the mobile phase for gradient elution. Finally, 3.65 g of the target product was obtained as white needle-like crystals. The yield was 88.48%.
[0037] Synthesis of polydiphenyltriphenylamine: in a pre-dried 250mL three-necked flask, add didi...
Embodiment 2
[0041]The polyditerphenylamine obtained in Example 1 is used as the active material of the positive electrode material, and the lithium-ion battery is prepared according to the following steps:
[0042] a) Weigh 1 part of binder powder (polyvinylidene fluoride: PVDF) and disperse it in 10 parts of N-methylpyrrolidone solvent, seal and stir, and slightly heat to obtain a bonding slurry.
[0043] b) Weigh 4 parts of acetylene black and 5 parts of prepared polymer powder and mix them evenly.
[0044] c) Pour the mixture in b) into the bonding slurry in a), then add appropriate N-methylpyrrolidone, stir and mix evenly to obtain a mixed slurry with moderate viscosity.
[0045] d) The slurry in c) is evenly coated on the aluminum foil, and then vacuum-dried in an oven at 60° C. for 24 hours to obtain a positive electrode sheet.
[0046] e) With the positive electrode sheet prepared in d) as the positive electrode, the metal lithium sheet as the negative electrode, 1mol / L LiPF 6 EC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com