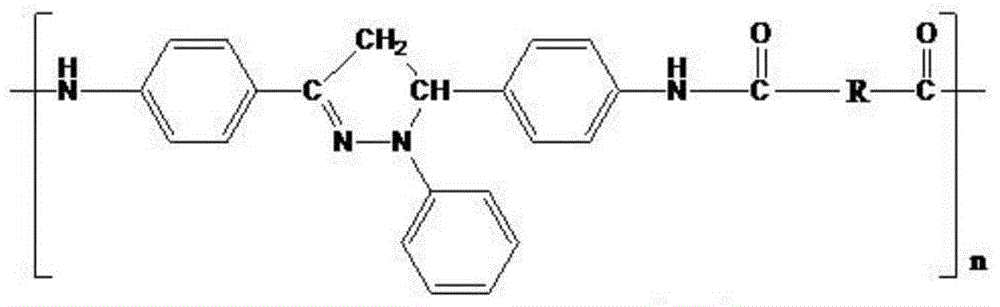
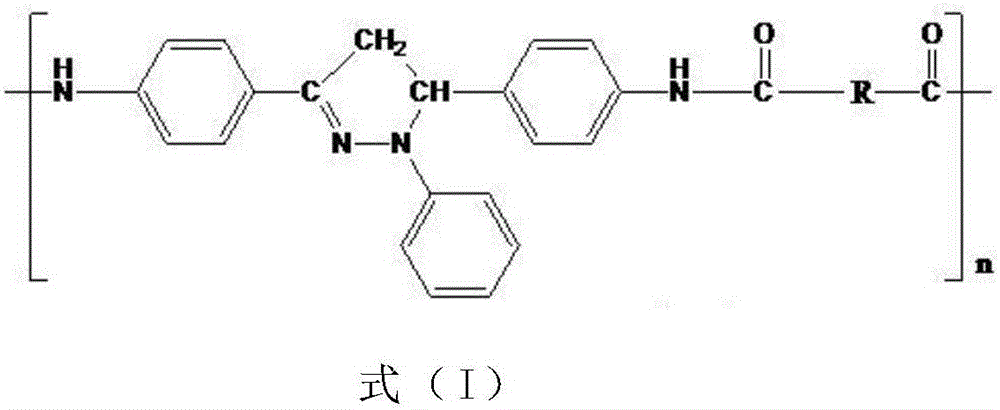
Organic blue light-emitting material and preparation method thereof
A blue light-emitting material, organic technology, applied in the direction of light-emitting materials, chemical instruments and methods, etc., can solve the problems of low glass transition temperature, device performance degradation, aggregation and crystallization, etc., and achieve stable physical and chemical properties and high glass transition Temperature, the effect of increasing the glass transition temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Synthesis of 1,3-Di-p-nitrophenylpropenone
[0033]Add 3.22g (0.021mol) of p-nitrobenzaldehyde and 20mL of absolute ethanol to a 100mL three-necked flask, then add 4mL of concentrated sulfuric acid, install a water flow condenser, control the temperature of the water bath at about 72 ° C, and stir the 3.30 g (0.02mol) p-nitroacetophenone was added to the reactor, and the reaction was continued for 12 hours after the addition was completed, neutralized to neutral with 20% sodium hydroxide solution after cooling, the solid was collected by suction filtration, washed with 50% ethanol, The crude product was obtained, recrystallized from absolute ethanol, and dried in vacuo to obtain an orange solid with a yield of 53.2%;
[0034] Synthesis of 1-phenyl-3,5-di-p-nitrophenylpyrazoline
[0035] Add 2.98g (0.01mol) of 1,3-di-p-nitrophenylpropenone and 25mL of ethylene glycol monoethyl ether into a three-necked flask equipped with a condenser tube, and then add 1.44g (0.013mol) ...
Embodiment 2
[0042] Synthesis of 1,3-Di-p-nitrophenylpropenone
[0043] Add 3.22g (0.021mol) of p-nitrobenzaldehyde and 20mL of absolute ethanol to a 100mL three-necked flask, then add 4mL of concentrated sulfuric acid, install a water flow condenser, control the temperature of the water bath at 72°C, and dissolve 3.30g of (0.02mol) p-nitroacetophenone is added in the reactor, continue to react for 12h after adding, neutralize to neutrality with 20% sodium hydroxide solution after cooling, collect solid by suction filtration, wash with 50% ethanol, obtain Crude. Recrystallized from absolute ethanol and dried in vacuo to obtain an orange solid with a yield of 53.2%;
[0044] Synthesis of 1-phenyl-3,5-di-p-nitrophenylpyrazoline
[0045] Add 2.98g (0.01mol) of 1,3-di-p-nitrophenylpropenone and 25mL of ethylene glycol monoethyl ether into a three-necked flask equipped with a condenser tube, and then add 1.44g (0.013mol) of phenylhydrazine, under nitrogen protection, 100 ° C constant tempera...
Embodiment 3
[0052] Synthesis of 1,3-Di-p-nitrophenylpropenone
[0053] Add 3.22g (0.021mol) of p-nitrobenzaldehyde and 20mL of absolute ethanol to a 100mL three-necked flask, then add 4mL of concentrated sulfuric acid, install a water flow condenser, control the temperature of the water bath at about 72 ° C, and stir the 3.30 g (0.02mol) p-nitroacetophenone was added to the reactor, and the reaction was continued for 12 hours after the addition was completed, neutralized to neutral with 20% sodium hydroxide solution after cooling, the solid was collected by suction filtration, washed with 50% ethanol, The crude product was obtained, recrystallized from absolute ethanol, and dried in vacuo to obtain an orange solid with a yield of 53.2%;
[0054] Synthesis of 1-phenyl-3,5-di-p-nitrophenylpyrazoline
[0055] Add 2.98g (0.01mol) of 1,3-di-p-nitrophenylpropenone and 25mL of ethylene glycol monoethyl ether into a three-necked flask equipped with a condenser tube, and then add 1.44g (0.013mol)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dispersity | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com