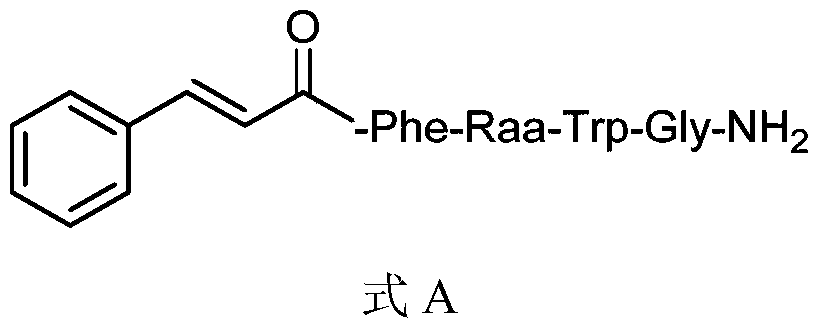
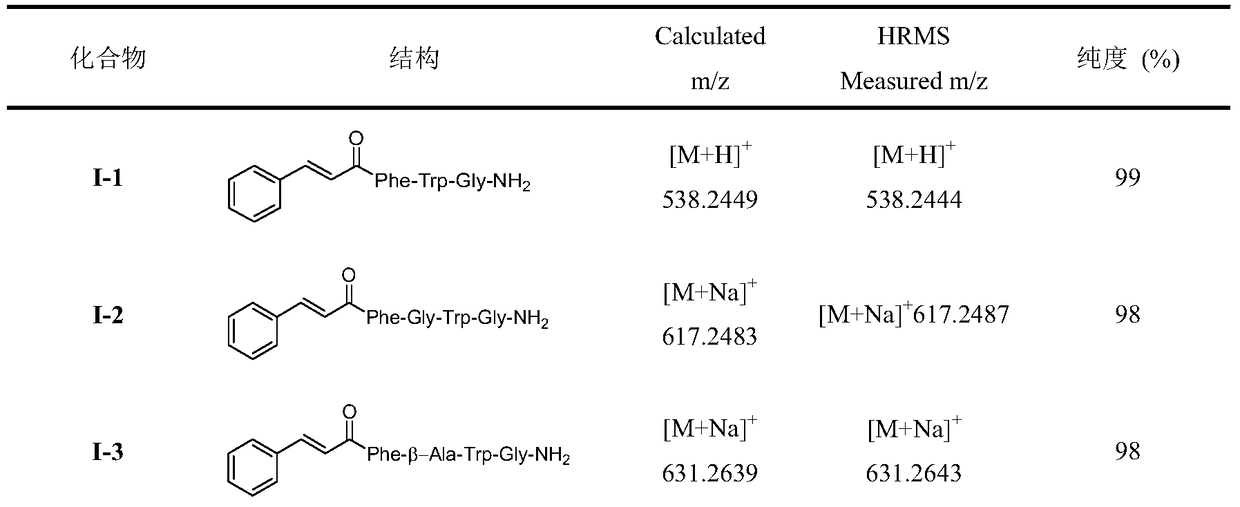
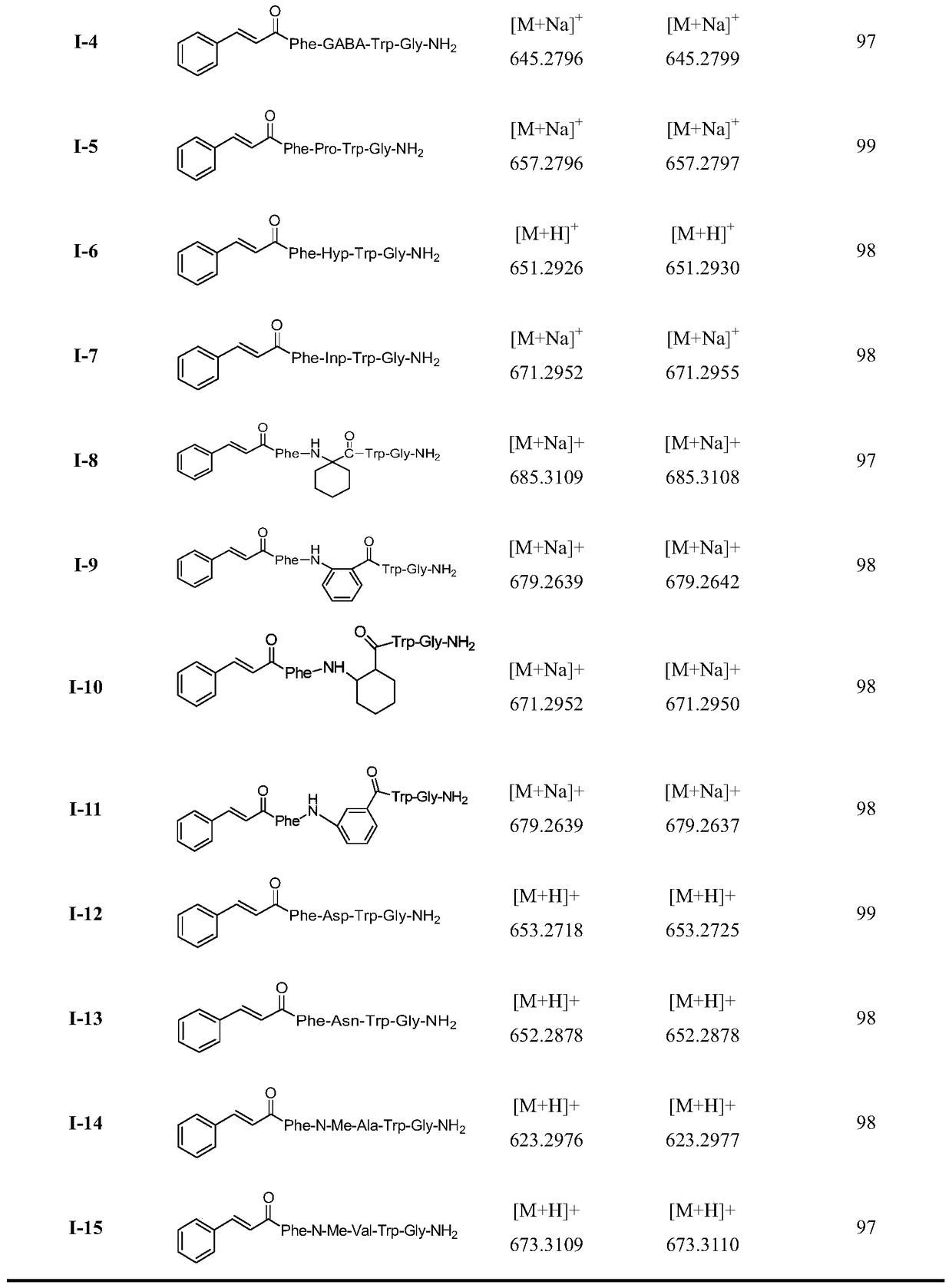
A class of insect kinin analogues and their applications
A technology of insect kinin and analogs, applied in the fields of application, peptides, insecticides, etc., can solve the problems of kinin analogs with not very prominent biological activity and large molecular weight, and achieve good in vitro inhibitory activity and good control effect , Insecticidal activity obvious effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: Preparation of 1-2 compound (when Raa is Gly)
[0022] (1) Resin activation: Weigh 500mg Rink Amide-AM resin, wash 4 times with DCM, add 5ml DCM to swell and activate for 3h, wash 4 times with DMF, add 20% piperidine DMF solution to cut for 20min, wash 4 times with 5ml DMF, 5ml DCM washed 4 times, Kaiser's reagent detection.
[0023] (2) Connect Gly: DMF wash 3 times, add 360mg Fmoc-Gly-OH (4 times the amount), 455mg HBTU (4 times the amount), 162mg HOBt (4 times the amount), 142ul DIEA (4 times the amount), dissolve in 5ml DMF, stirred at room temperature for 2h, washed 4 times with 5ml DMF, cut by adding 20% piperidine DMF solution for 20min, washed 4 times with 5ml DMF, washed 4 times with 5ml DCM, detected by Kaiser's reagent.
[0024] (3) Receive Trp(Boc): wash with DMF 3 times, add 630mg Fmoc-Trp(Boc)-OH (4 times the amount), 455mg HBTU (4 times the amount), 162mg HOBt (4 times the amount), 142ul DIEA (4 times the amount) double amount), dissolve...
Embodiment 2
[0034] Embodiment 2: the biological activity of formula A target compound to aphids
[0035] The insecticidal activity of the target compound of formula A to aphids is determined by the leaf-dipping method. The target compound was made into a 2000 mg / L assay solution. Then use a 1-5ml pipette gun to take 1ml of acetone and add it to a weighing bottle, add 9ml of an aqueous solution containing 0.1% Triton X-100, and mix well to obtain a 200mg / L measurement solution, and then use 0.1% Triton X-100 Dilute step by step with the aqueous solution of X-100, and mix thoroughly to obtain the desired concentration. Cultivate soybean aphids and soybean leaves that have not been exposed to any pesticides indoors, use a puncher with a diameter of 15mm to punch out leaves of appropriate size, immerse them in the diluted medicinal solution for 15 seconds, and take them out to dry. Then the leaves were put into a bioassay plate, 1% agar was added to the bottom to moisturize, 20±3 soybean ap...
Embodiment 3
[0042] Embodiment 3: the biological activity of formula A target compound to plant pathogenic bacteria
[0043] The indoor and in vitro inhibitory activity of the compounds against plant pathogenic bacteria was determined by the turbidity-ELISA method.
[0044] The target bacteria tested were 7 common pathogens, including Phytophthora capsici, Fusarium fujikuroi, Bipolaris maydis, Colletotrichum gloeosporioides, Botrytis cinerea Botrytis cinerea, Alternaria solani and Sporisorium reilianum.
[0045] Compounds to be tested were formulated with DMSO into 10 4 mg / L mother solution, and then diluted with sterile water to form a series of gradient concentration drug solutions, and placed at 4°C for later use.
[0046] Cultivation of target bacteria: Inoculate the strains that are prone to spore production (bakanae rice bacterium, pepper early blight bacterium, and corn spot bacterium) on the optimal medium plate and carry out spore production culture. After it produces a large nu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com