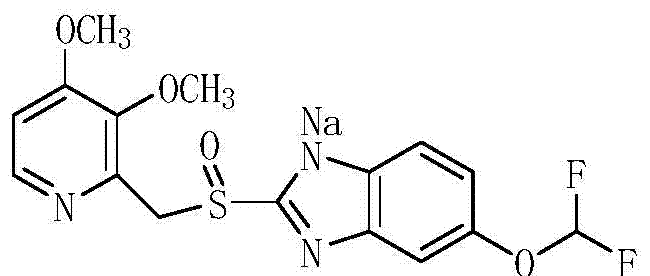
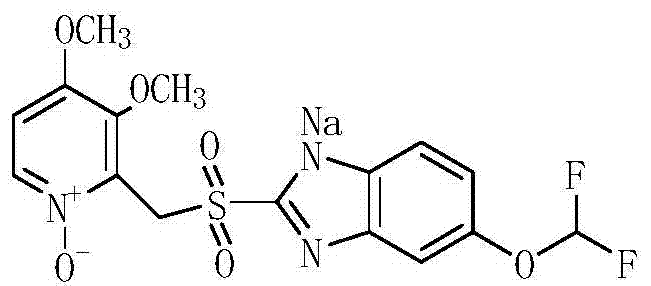
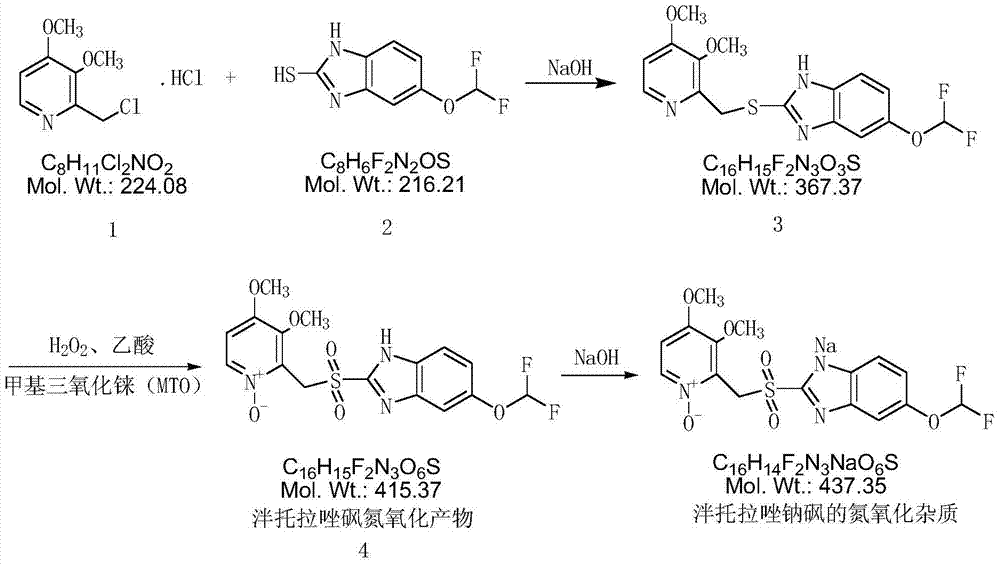
A kind of preparation method of pantoprazole sodium sulfone nitrogen oxide impurity
A technology for pantoprazole sodium and oxidized impurities, applied in the field of medicine, can solve problems such as being difficult to remove, and achieve the effects of cheap raw materials, high yield and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Add 10g 2-chloromethyl-3,4-dimethoxypyridine hydrochloride (compound 1), 10g 5-difluoromethoxy-2-mercapto-1H-benzimidazole to the reaction flask (Compound 2), 100ml of dichloromethane, stirred, dropwise added 130g of 10% sodium hydroxide solution, stirred at 20-30°C for 2h, allowed to stand for layers, washed the dichloromethane layer twice with 30ml of water each time, Then underpressure distillation obtains yellow oil 16.6g (intermediate 3);
[0031] (2) Dissolve the yellow oil in step (1) with 40ml acetic acid, add 15.8g 50% hydrogen peroxide, 0.68g methyl rhenium trioxide (MTO), heat up to 95°C, and react for 6h; after the reaction is completed, pour the reaction solution Pour it into 100ml of water, adjust the pH to neutral with 10% sodium hydroxide solution, extract the aqueous layer with 50ml (each amount) of dichloromethane three times, and distill the dichloromethane under reduced pressure to obtain a yellow oily substance. Dissolve the oil in 20ml of tolu...
Embodiment 2
[0034] (1) Dissolve the yellow oil in step (1) of Example 1 with 45ml of acetic acid, add 18.2g of 50% hydrogen peroxide, 0.88g of methyl rhenium trioxide (MTO), heat up to 95°C, and react for 7h; after the reaction is completed, The reaction solution was poured into 100ml of water, and the pH was adjusted to be neutral with 10% sodium hydroxide solution. The aqueous layer was extracted three times with 50ml (each dosage) of dichloromethane, and the dichloromethane was distilled under reduced pressure to obtain a yellow oil. Dissolve the oil in 23ml of toluene, add 175ml of methyl tert-butyl ether, stir for 1h, and filter with suction to obtain 14.1g of a light yellow solid (Intermediate 4), with a purity of 97.5% and a molar yield of 76.0%;
[0035] (2) Dissolve 14.1g of intermediate 4 in 23ml of acetone, add 8.5g of 20% sodium hydroxide solution, cool down to 0°C and stir for 30min, and suction filter to obtain 13.2g of white solid with a purity of 98.4% and a molar yield of ...
Embodiment 3
[0036] (1) The yellow oil of step (1) of Example 1 was dissolved with 50ml of acetic acid, 22.0g of 50% hydrogen peroxide was added, 1.0g of methyl rhenium trioxide (MTO), the temperature was raised to 95°C, and the reaction was carried out for 8h; after the reaction was completed, The reaction solution was poured into 100ml of water, and the pH was adjusted to be neutral with 10% sodium hydroxide solution. The aqueous layer was extracted three times with 50ml (each dosage) of dichloromethane, and the dichloromethane was distilled under reduced pressure to obtain a yellow oil. Dissolve the oil in 25ml of toluene, add 180ml of methyl tert-butyl ether, stir for 1h, and filter with suction to obtain 14.6g of a light yellow solid (Intermediate 4), with a purity of 97.8% and a molar yield of 78.6%;
[0037] (2) Dissolve 14.6g of intermediate 4 in 25ml of acetone, add 8.8g of 20% sodium hydroxide solution, cool down to 0°C and stir for 30min, and filter with suction to obtain 13.8g o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com