Osmotic pump controlled-release preparation containing loxoprofen sodium and preparation method thereof
An osmotic pump controlled release, loxoprofen sodium technology, applied in the directions of anti-inflammatory agents, pill delivery, non-central analgesics, etc., can solve the problems of easy side effects, poor absorption, and many times of administration. The effect of reducing gastrointestinal irritation and plasma concentration fluctuations, reducing the number of administrations, and improving patient compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
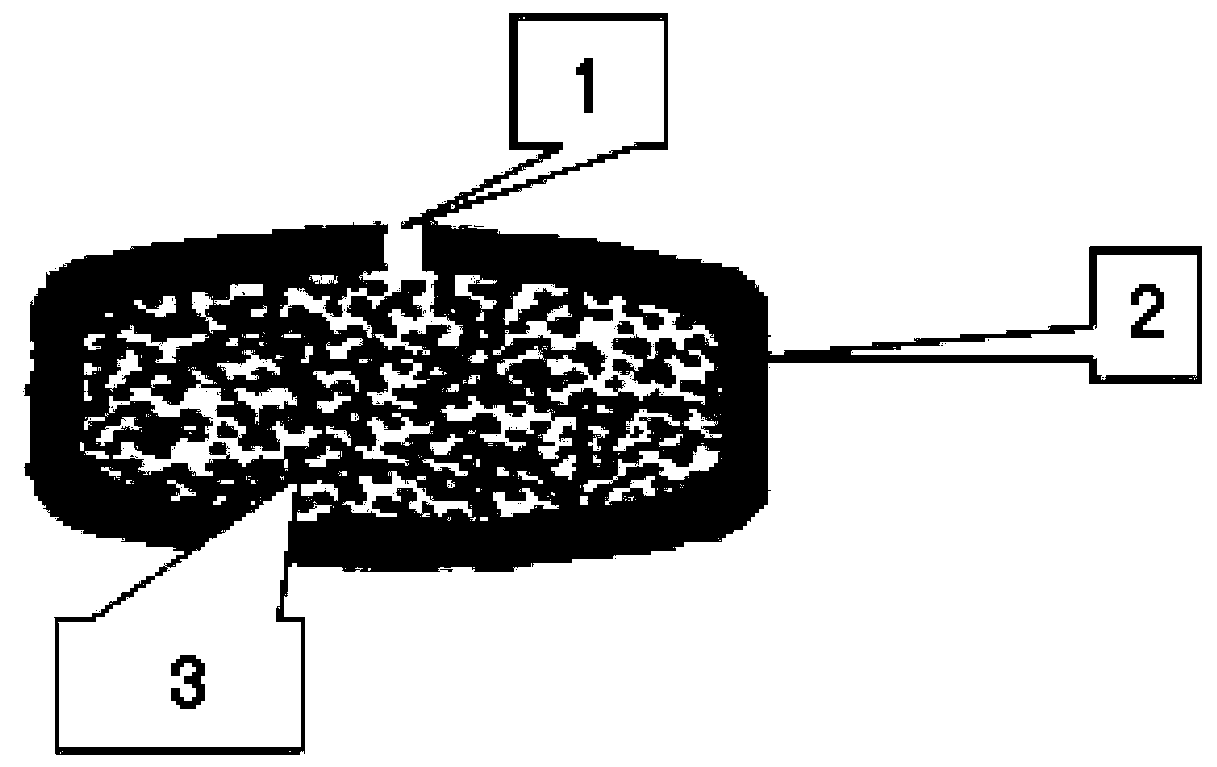
[0030] Such as figure 2 As shown, this embodiment provides an osmotic pump controlled-release preparation containing loxoprofen sodium, which includes a tablet core 3 and a semipermeable membrane 2 with a drug release hole 1. The tablet core is made of the following raw materials by weight percentage Ingredients: Loxoprofen sodium (calculated as anhydrous) 90g, sodium chloride 13g, lactose 4g, microcrystalline cellulose 103g, ethanol 25g, magnesium stearate 3g;
[0031] The semi-permeable membrane is made of the following raw materials according to weight percentage: 40.5g of cellulose acetate, 2.59g of polyethylene glycol, 20ml of water, and 1000ml of acetone.
[0032] The preparation method of the above-mentioned osmotic pump control preparation containing loxoprofen sodium is as follows:
[0033] (1) Pass the loxoprofen sodium, filler, osmotic pressure enhancer, release regulator, and binder through a 60-mesh sieve, and mix them according to the above weight percentage, then add...
Embodiment 2
[0038] This embodiment provides an osmotic pump controlled-release preparation containing loxoprofen sodium, which includes a tablet core and a semipermeable membrane with drug release holes, and the tablet core is made of the following raw materials by weight percentage: Sodium (calculated as anhydrous) 90g, sodium chloride 70g, microcrystalline cellulose 20g, polyvinylpyrrolidone 16g, ethanol 20g, magnesium stearate 4g;
[0039] The semi-permeable membrane is made of the following raw materials according to weight percentage: 36.3 g of cellulose acetate, 3.16 g of polyethylene glycol, 20 ml of water, and 1100 ml of acetone.
[0040] The preparation method of the above-mentioned osmotic pump control preparation containing loxoprofen sodium is as follows:
[0041] (1) Pass loxoprofen sodium, fillers, osmotic pressure enhancers, release regulators, and adhesives through a 40-mesh sieve, and mix them according to the above weight percentages, then add ethanol to make a soft material, a...
Embodiment 3
[0046] This embodiment provides an osmotic pump controlled-release preparation containing loxoprofen sodium, which includes a tablet core and a semipermeable membrane with drug release holes, and the tablet core is made of the following raw materials by weight percentage: Sodium (calculated as anhydrous) 90g, sodium chloride 41g, microcrystalline cellulose 45g, polyvinylpyrrolidone 9g, hydroxypropylmethylcellulose 35g, ethanol 22g, magnesium stearate 5g;
[0047] The semi-permeable membrane is made of the following raw materials according to weight percentage: 36.0g cellulose acetate, 3.44g polyethylene glycol, 24ml water, 1200ml acetone
[0048] The preparation method of the above-mentioned osmotic pump control preparation containing loxoprofen sodium is as follows:
[0049] (1) Pass the loxoprofen sodium, filler, osmotic pressure enhancer, release regulator, and adhesive through an 80-mesh sieve, and mix them according to the above weight percentage, then add ethanol to make a soft...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com