Preparation method of cefcapene pivoxil hydrochloride
A technology of cefcapene pivoxil hydrochloride and hydrochloric acid, which is applied in the field of medicine and chemical industry, can solve the problems of unsuitability for large-scale production, low yield, complicated operation, etc., and achieve the advantages of simple operation, high reaction yield, and high reaction yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
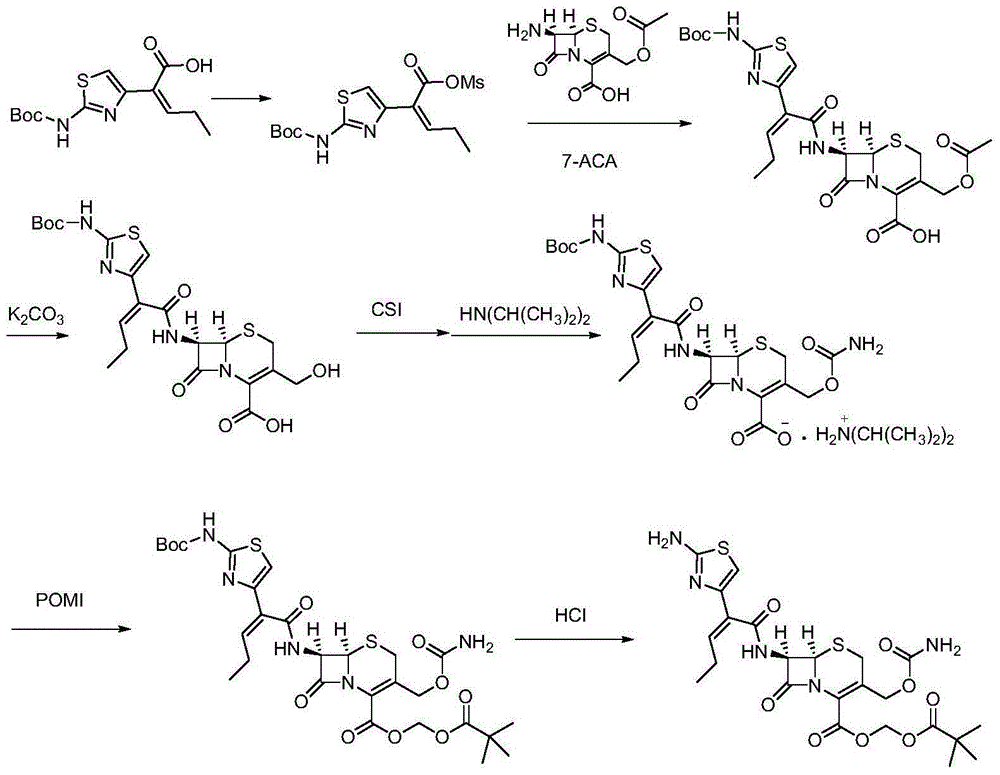
[0034] The preparation method of cefcapene pivoxil hydrochloride comprises the steps:
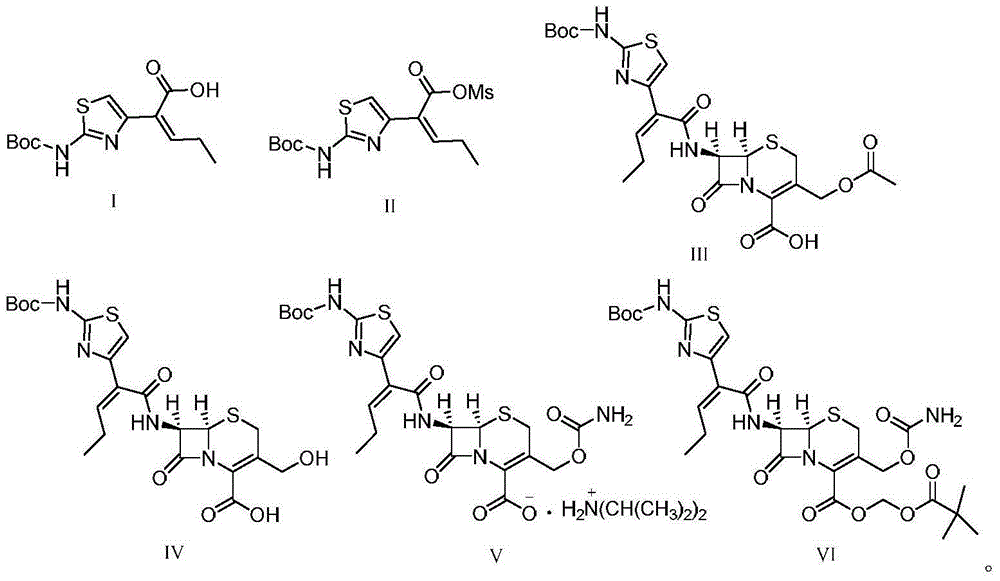
[0035] 1) 29.8 g (100 mmol) of the compound represented by formula I ((Z)-2-(2-tert-butoxycarbonylaminothiazol-4-yl)-2-pentenoic acid)) was stirred and dissolved in 90 mL of pyridine, Add 16 g (140 mmol) of methanesulfonyl chloride at -10°C, stir and react for 2 hours, then filter to obtain a liquid containing the compound represented by formula (II), store it at -15°C, and set aside;
[0036]2) First mix 4.6g of proline, 12.1g (120mmol) of diisopropylamine and 23.1g (85mmol) of 7-ACA in 100ml of methanol and lower the temperature to 10°C, then mix the product obtained in step 1) containing the formula II The liquid of the compound was dripped into the reaction for 2h, naturally rose to room temperature, adjusted the pH of the solution to 4 with 2mol / L hydrochloric acid, extracted, combined the organic phases, concentrated under reduced pressure, recrystallized from methanol, and dried to o...
Embodiment 2
[0042] The preparation method of cefcapene pivoxil hydrochloride comprises the steps:
[0043] 1) 29.8 g (100 mmol) of the compound ((Z)-2-(2-tert-butoxycarbonylaminothiazol-4-yl)-2-pentenoic acid) represented by formula I was dissolved in 80 ml of pyridine with stirring, Add methanesulfonyl chloride at -15°C, stir and react for 1-2 hours, then filter to obtain a liquid containing the compound represented by formula (II), store it at -10°C, and set aside;
[0044] 2) First, mix 4.4g of proline, 10.1g (100mmol) of diisopropylamine and 24.5g (90mmol) of 7-ACA in 100m methanol and cool down to 15°C, then mix the product obtained in step 1) containing the formula II The liquid of the compound was dropped into the reaction for 1 hour, and naturally rose to room temperature, and the pH of the solution was adjusted to 4 with 2mol / L hydrochloric acid, extracted with dichloromethane, the organic phases were combined, concentrated under reduced pressure, recrystallized from methanol, an...
Embodiment 3
[0050] The preparation method of cefcapene pivoxil hydrochloride comprises the steps:
[0051] 1) 29.8 g (100 mmol) of the compound represented by formula I ((Z)-2-(2-tert-butoxycarbonylaminothiazol-4-yl)-2-pentenoic acid)) was stirred and dissolved in 120 ml of pyridine, Add 14.9 g (130 mmol) of methanesulfonyl chloride at 0°C, stir and react for 2 hours, then filter to obtain a liquid containing the compound represented by formula (II), store it at -15-0°C, and set aside;
[0052] 2) First, mix 3.3g of proline, 11.1g (110mmol) of diisopropylamine (110mmol) and 21.8g (80mmol) of 7-ACA in 100m methanol and cool down to 12°C. The liquid of the compound was dropped into the reaction for 2 hours, and naturally rose to room temperature, and the pH of the solution was adjusted to 4 with 2mol / L hydrochloric acid, extracted with dichloromethane, the organic phases were combined, concentrated under reduced pressure, recrystallized from methanol, and dried to obtain the compound shown ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com