Method for preparing anti-human R47H-TREM2 mutant human monoclonal antibody
A human monoclonal antibody and mutant technology, applied in the field of biomedicine, can solve the problems of difficulty and impracticality of monoclonal antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0050] A method for preparing a human monoclonal antibody against a human R47H-TREM2 mutant, comprising the steps of:
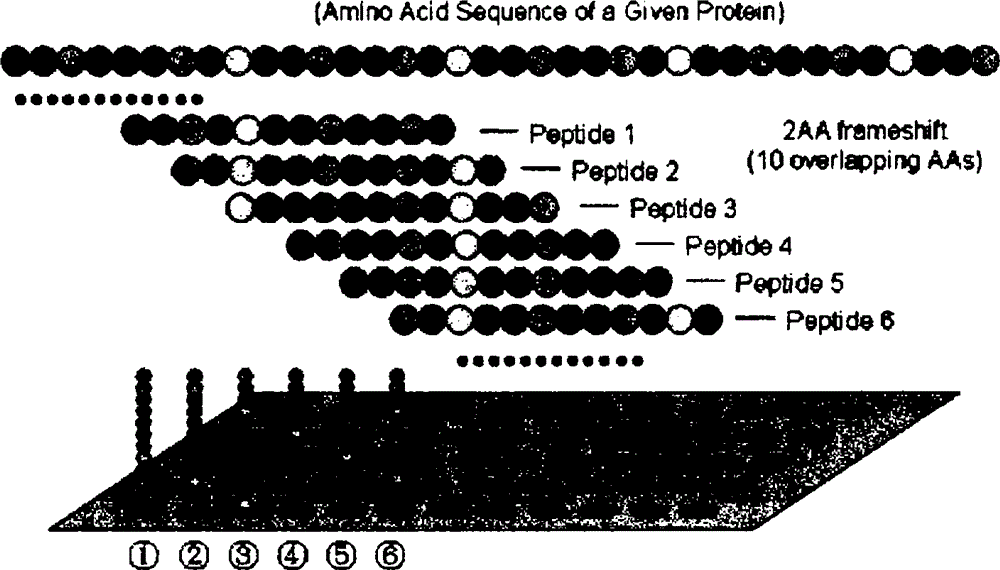
[0051] 1) Synthesis of polypeptide antigen
[0052] 201 R47H-TREM2 polypeptides of 6-15 aa were artificially synthesized to make the epitope surround the mutation target of TREM2 gene and expand to both sides centered on R47 of TREM2. Each polypeptide sequence was staggered by 1-2 aa; then HPLCC-18 reversed Column purified and freeze-dried;
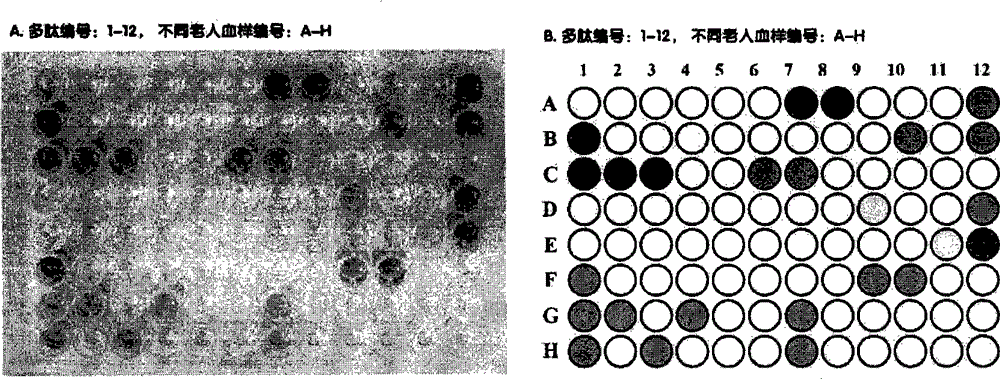
[0053] 2) Preparation of R47H-TREM2 mutant peptide antigen array PeptideArray
[0054] Use 96 or 184-well ELISA plates, dilute the polypeptide antigen to 1-10 μg / ml with coating buffer 0.05mol / L carbonate buffer, add 0.1ml to each well, overnight at 4°C, wash 3 times the next day and use Wet box 4C preservation;
[0055] 3) Collection of CD22+ B cells
[0056] Collect peripheral blood from 60-80-year-old healthy elderly and AD patients, 15mL / person, and perform density gradient centrifugation with FicollHypaque reage...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com