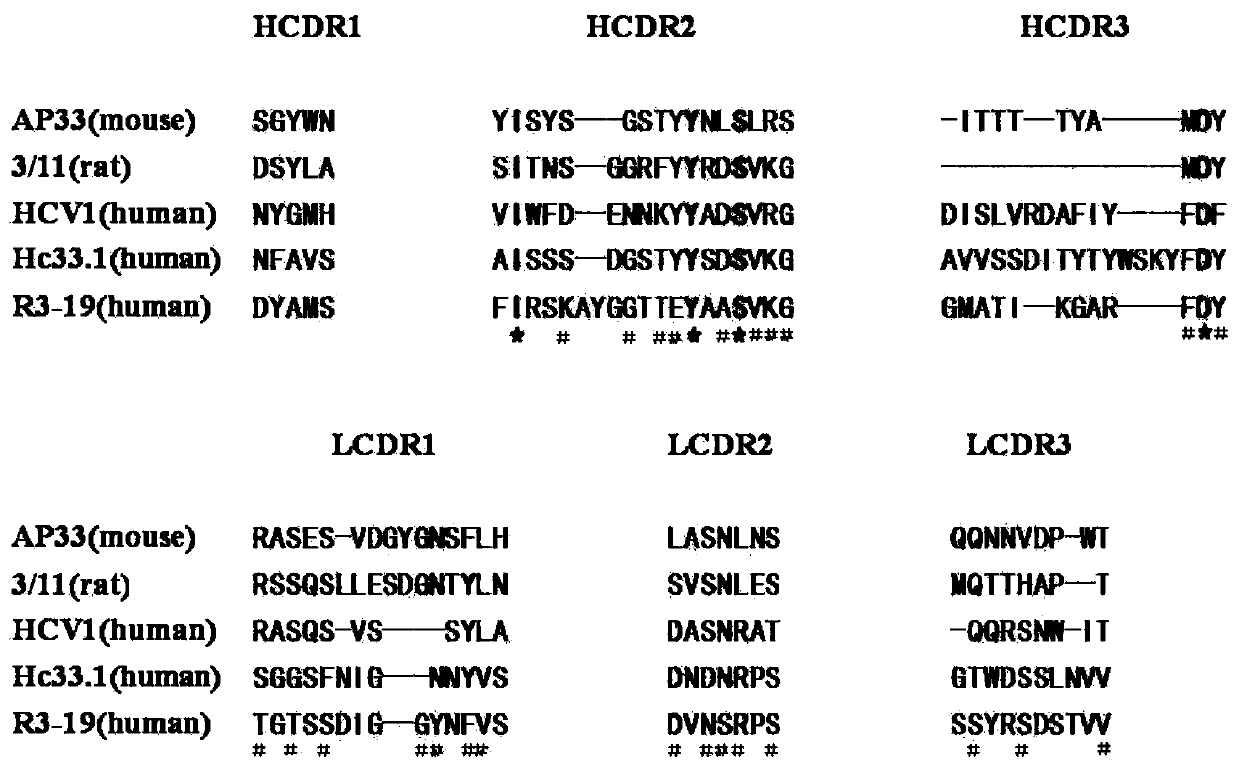
Anti-multi-subgenotype HCV antibody gene r3-19 and its application
A genotype and antibody technology, applied in the field of biochemistry, can solve the problems of differences in the neutralization ability of different genotypes, and achieve the effect of extensive binding characteristics and neutralization ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
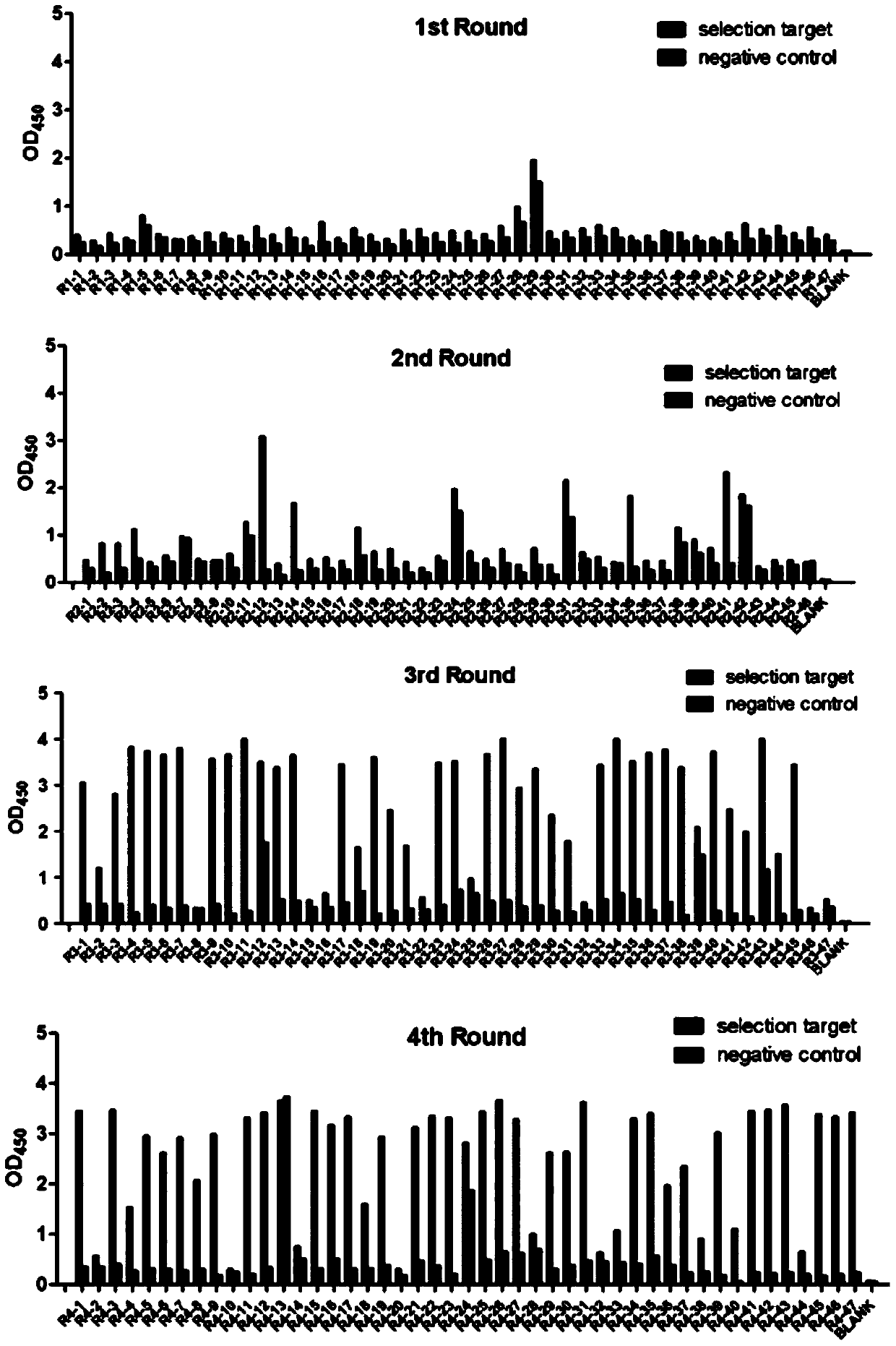
Examples
Embodiment 1
[0022] Embodiment 1, primer design
[0023] According to the report of John McCafferty, the mRNA gene-specific reverse transcription primers for human IgG, IgM heavy chain constant region and κ, λ light chain constant region, as well as PCR amplification primers for heavy chain and light chain variable region were designed (McCafferty J, Griffiths AdFau- Winter G, Winter G Fau-Chiswell DJ, Chiswell DJ. Phage antibodies: filamentous phage displaying antibody variable domains. Nature. 1990; 348(6301): 552-4.). In order to facilitate insertion into the T7 phage vector, EcoR I and Hind III restriction sites were added to the 5' end of the forward primer for the variable region of the light chain (VL) and the 5' end of the reverse primer for the variable region of the heavy chain (VH). In order to generate the VL-linker-VH gene fragment, the PCR-amplified VL and VH fragments were connected by overlapping extension PCR, and the reverse overlaps were added to the 5' end of the VL rev...
Embodiment 2
[0028] Example 2, mRNA extraction and cDNA synthesis
[0029] Combining RNA / DNA Stabilization Reagent for Blood / Bone Marrow (Roche) and mRNA isolation kit for blood / bone marrow (Roche), using the magnetic bead method to directly isolate and extract mRNA from peripheral blood leukocytes, operate according to the instructions, and measure the concentration of extracted mRNA and A260 / A280 values. The results showed that an average of 130 ng of mRNA was obtained per milliliter of anticoagulated peripheral blood cells of the patient, and the A260 / A280 value was 1.90-2.00, indicating that the isolated and extracted mRNA can be used for library construction, and then stored at -80°C for later use. Take 50ng of mRNA extracted from all samples as a template, respectively use human IgG, IgM heavy chain constant region, κ, λ light chain constant region mRNA gene-specific reverse transcription primers, II FirstStrand cDNA Synthesis Kit (NEB) synthesized cDNA, combined cDNA, and stored a...
Embodiment 3
[0030] Embodiment 3, single-chain variable region fragment (scFv) amplification
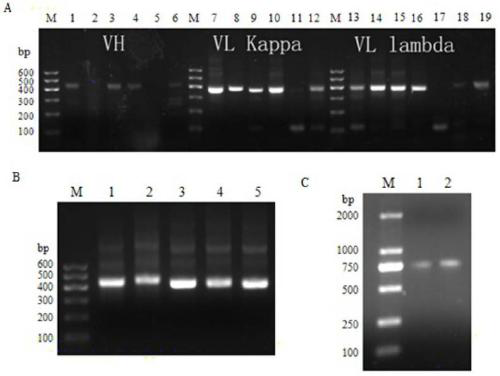
[0031] The heavy chain and light chain variable region genes were amplified by PCR, and the specific operation method was as follows: the heavy chain and the reverse primers for the variable region of the κ and λ light chains were premixed at equimolar concentrations, and the final concentration was 10 μM. In 50μL PCR volume, each contains 25pmoL of single upstream primer and premixed reverse primer, 2μL of cDNA, 10μL of 5×Reaction Buffer, 10μL of 5×High GC Enhancer, 1μL of 10mmol dNTP, High-Fidelity DNA Polymerase (NEB) 1 unit. Reaction conditions: 98°C pre-denaturation for 30 seconds, 98°C denaturation for 10 seconds, 60°C annealing for 30 seconds, 72°C extension for 30 seconds, a total of 25 cycles; finally 72°C extension for 2 minutes. The PCR product was subjected to agarose gel electrophoresis, and was purified by High Pure PCR Product Purification Kit (Roche). The electrophoresis results...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap