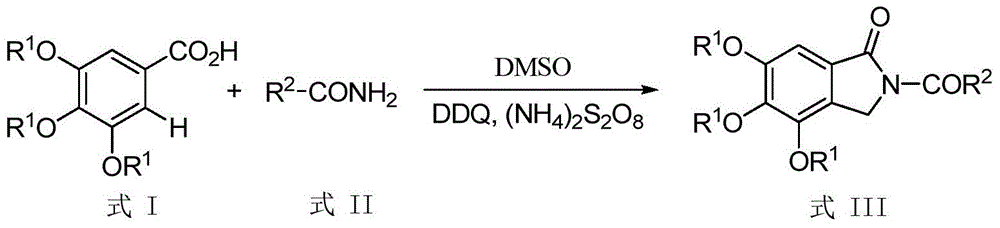One-step synthesis method of 2-acyl substituted isoindoline-1-one compounds
A technology of ketone compound and isoindoline is applied in the field of 2-acyl substituted isoindoline, which can solve the problems of harsh reaction conditions and the use of precious metals, and achieve simple operation and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
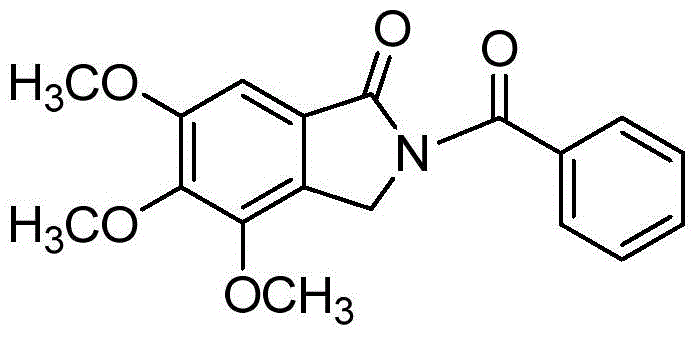
[0014] Taking the preparation of 4,5,6-trimethoxy-2-benzoylisoindolin-1-one with the following structural formula as an example, the raw materials used and the preparation method are as follows:
[0015]
[0016] Add 0.0212g (0.1mmol) 3,4,5-trimethoxybenzoic acid, 0.0255g (0.2mmol) benzamide, 0.0579g (0.25mmol) 2,3-dichloro-5 ,6-dicyano-p-benzoquinone (DDQ), 0.0360g (0.15mmol) (NH 4 ) 2 S 2 o 8 , 30μL (0.4mmol) DMSO, 0.6mL 1,2-dichloroethane, under the protection of argon in a closed system, stirred and reacted at 130°C for 24 hours, cooled to room temperature after the reaction was completed, filtered through a column chromatography silica gel column, and used Separation by thin-layer chromatography yielded 4,5,6-trimethoxy-2-benzoylisoindolin-1-one with an isolated yield of 68%, and the structural characterization data are as follows:
[0017] 1 H NMR (400MHz, CDCl 3 ): δ(ppm)=7.68(d, J=7.0Hz, 2H), 7.57~7.52(m, 1H), 7.45(t, J=7.5Hz, 2H), 7.09(s, 1H), 4.99(s , 2H), ...
Embodiment 2
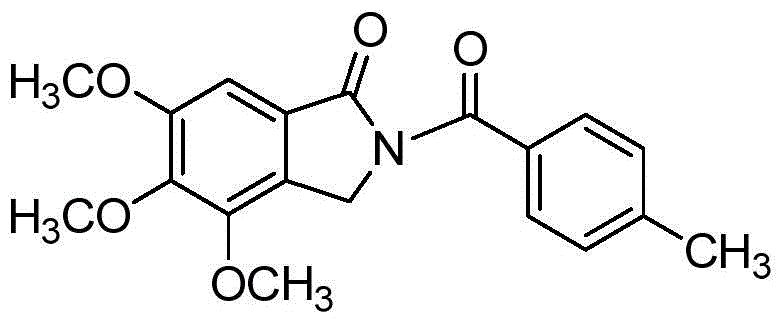
[0019] Taking the preparation of 4,5,6-trimethoxy-2-p-tolylisoindolin-1-one with the following structural formula as an example, the raw materials used and the preparation method are as follows:
[0020]
[0021] In Example 1, the benzamide used was replaced with equimolar p-toluamide, and the other steps were the same as in Example 1 to obtain 4,5,6-trimethoxy-2-p-toluylbenzoyl Isoindolin-1-one, its isolated yield is 64%, and the structural characterization data are as follows:
[0022] 1 H NMR (600MHz, CDCl 3 ): δ (ppm) = 7.67 (d, J = 7.9Hz, 2H), 7.34 (d, J = 7.9Hz, 2H), 7.15 (s, 1H), 5.01 (s, 2H), 4.13 (s, 3H ), 4.01(s, 3H), 3.99(s, 3H), 2.48(s, 3H); 13 C NMR (150MHz, acetone-d 6 ): δ (ppm) = 174.6, 171.2, 160.7, 153.7, 151.8, 147.0, 137.6, 134.4, 133.3, 132.1, 131.6, 107.3, 65.6, 65.2, 61.1, 51.4, 25.9; HRMS (ESI) m / z: C 18 h 17 NO 5 , [M+Na] + The theoretical value is 364.1161, and the measured value is 364.1158.
Embodiment 3
[0024] Taking the preparation of 4,5,6-trimethoxy-2-(4-n-butylbenzoyl)isoindolin-1-one with the following structural formula as an example, the raw materials used and the preparation method are as follows:
[0025]
[0026] In Example 1, the benzamide used was replaced with equimolar p-n-butylbenzamide, and the other steps were the same as in Example 1 to obtain 4,5,6-trimethoxy-2-(4-n-butyl Benzoyl) isoindolin-1-one, its isolated yield is 60%, and the structural characterization data are as follows:
[0027] 1 H NMR (400MHz, acetone-d 6): δ (ppm) = 7.63 (d, J = 7.8Hz, 2H), 7.30 (d, J = 7.8Hz, 2H), 7.09 (s, 1H), 4.95 (s, 2H), 4.07 (s, 3H ), 3.94(s, 3H), 3.93(s, 3H), 2.72(t, J=7.7Hz, 2H), 1.70~1.63(m, 2H), 1.43~1.38(m, 2H), 0.95(d, J=7.3Hz, 3H); 13 C NMR (150MHz, acetone-d 6 ): δ (ppm) = 174.7, 171.2, 160.7, 153.7, 151.9, 137.8, 134.4, 134.3, 132.7, 132.1, 131.6, 107.2, 65.6, 65.2, 61.1, 51.4, 40.5, 38.5, 27.3, 18.5; ) m / z: C 22 h 25 NO 5 , [M+Na] + The theoretical...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com