Process for preparing epichlorohydrin
A technology of epichlorohydrin and chloropropene, applied in the direction of organic chemistry, etc., can solve the problems of low utilization rate of raw materials, easy decomposition of peroxide when heated, and explosion hazard, etc., achieve fast heat removal, easy temperature control, and prevent reaction from getting out of control Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
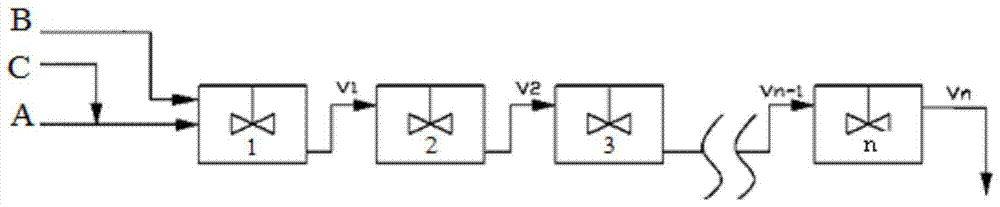
[0038] use figure 1 As shown in the flow process, 2 reactors are used in series, and the fresh chloropropene with a flow rate of 0.5 kg / h is mixed with the circulating chloropropene of 1.2 kg / h and the homogeneous molybdenum-based catalyst with a flow rate of 3.3 g / h. Enter. The flow rate is 2 kg / h. After the ethylbenzene solution containing 50% ethylbenzene hydroperoxide is mixed with 1 kg / h circulating ethylbenzene, it enters from the top inlet of the reactor 1.
[0039] The weight ratio of molybdenum to ethylbenzene hydroperoxide is 0.00005, and allyl chloride: ethylbenzene hydroperoxide=3 (molar ratio).
[0040] The reaction temperature of the reactor 1 is 30° C., the reaction pressure is 0.1 MPa, and the conversion rate of ethylbenzene hydroperoxide is 40%.
[0041] The reaction temperature of the reactor 2 is 50° C., the reaction pressure is 0.1 MPa, and the conversion rate of ethylbenzene hydroperoxide is 100%.
[0042] The stream containing epichlorohydrin, chloropr...
Embodiment 2
[0048] use figure 1 As shown in the flow process, 4 reactors are used in series, and the fresh chloropropene with a flow rate of 0.5 kg / h is mixed with the circulating chloropropene of 2 kg / h and the homogeneous molybdenum-based catalyst with a flow rate of 6.7 g / h. Enter. The flow rate is 2 kg / hour. After the cumene solution containing 50% cumene hydroperoxide is mixed with 1 kg / hour circulating cumene, it enters from the top inlet of the reactor 1.
[0049] The weight ratio of molybdenum and cumene hydroperoxide is 0.0001, and allyl chloride: cumene hydroperoxide=5 (mol ratio).
[0050] The reaction temperature of the reactor 1 is 30° C., the reaction pressure is 0.3 MPa, and the conversion rate of cumene hydroperoxide is 30%.
[0051] The reaction temperature of the reactor 2 is 40° C., the reaction pressure is 0.3 MPa, and the conversion rate of cumene hydroperoxide is 60%.
[0052] The reaction temperature of the reactor 3 is 50° C., the reaction pressure is 0.3 MPa, a...
Embodiment 3
[0060] use figure 1 As shown in the flow process, 5 reactors are used in series, and the fresh chloropropene with a flow rate of 0.5 kg / h is mixed with the circulating chloropropene of 3 kg / h and the homogeneous molybdenum-based catalyst with a flow rate of 20 g / h. Enter. The flow rate is 2 kg / h. After the ethylbenzene solution containing 50% ethylbenzene hydroperoxide is mixed with 1 kg / h circulating ethylbenzene, it enters from the top inlet of the reactor.
[0061] The weight ratio of molybdenum to ethylbenzene hydroperoxide is 0.0003, and allyl chloride: ethylbenzene hydroperoxide=7 (molar ratio).
[0062] The reaction temperature of the reactor 1 is 30° C., the reaction pressure is 0.5 MPa, and the conversion rate of ethylbenzene hydroperoxide is 35%.
[0063] The reaction temperature of the reactor 2 is 50° C., the reaction pressure is 0.5 MPa, and the conversion rate of ethylbenzene hydroperoxide is 55%.
[0064] The reaction temperature of the reactor 3 is 90° C., t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com