Preparation method of copper, cobalt and sulfur micropowder
A copper-cobalt-sulfur ultra-fine powder technology, applied in electrical components, electrochemical generators, battery electrodes, etc., can solve the problems of no copper-cobalt-sulfur ultra-fine powder reports, limited copper-cobalt-sulfur research reports, cumbersome experimental procedures, etc. , to achieve the effects of easy large-scale industrial production, large reversible charge and discharge capacity, and simple product separation operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A preparation method of copper cobalt sulfur superfine powder, it comprises the following steps:
[0038] (1) Weigh 0.1997g copper acetate hydrate (Cu(CH 3 COO) 2 ·H 2 O, purity ≥99.0%), 0.4982g of cobalt acetate hydrate (Co(CH 3 COO) 2 4H 2 O, purity ≥99.5%) and 0.3045g of thiourea (CH 4 N 2 S, purity ≥ 99.0%), dissolved in 60ml of ethylene glycol, stirred to form a solution;
[0039] (2) Transfer the solution obtained in step (1) to a 100ml stainless steel reaction kettle lined with polytetrafluoroethylene, and react for 12 hours at 200°C to obtain a black product after the reaction;
[0040] (3) Wash the obtained black product with absolute ethanol, centrifuge and wash it three times, and then dry it under vacuum at 80°C for 12 hours, so as to obtain black copper-cobalt-sulfur ultrafine powder.
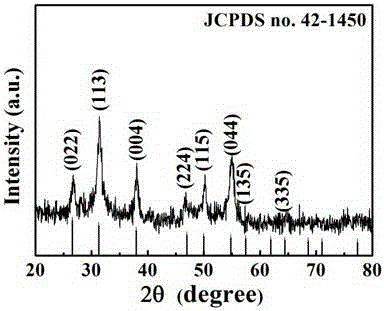
[0041] As attached to the manual Figure 1-7 As shown, the obtained copper-cobalt-sulfur ultrafine powder is tested and it can be seen that: the copper-cobalt-sulfu...
Embodiment 2
[0043] A preparation method of copper cobalt sulfur superfine powder, it comprises the following steps:
[0044] (1) Weigh 0.2491g copper nitrate hydrate (Cu(NO 3 ) 2 ·3H 2 O, purity ≥99.5%), 0.5821g of cobalt nitrate hydrate (Co(NO 3 ) 2 ·6H 2 O, purity ≥99.0%) and 0.3045g of thiourea (CH 4 N 2 S, purity ≥ 99.0%), dissolved in 60ml of ethanol, stirred to form a solution;
[0045] (2) Transfer the solution obtained in step (1) to a 100ml stainless steel reactor lined with polytetrafluoroethylene, and react at 220°C for 6 hours;
[0046] (3) The product obtained in step (2) was washed with absolute ethanol and centrifuged for three times, and then dried under vacuum at 80°C for 12 hours to obtain black copper-cobalt-sulfur ultrafine powder.
[0047] As attached to the manual Figure 8 As shown, the copper-cobalt-sulfur ultrafine powder prepared in this example is a nano-flower morphology composed of nano-sheets.
Embodiment 3
[0049] A preparation method of copper cobalt sulfur superfine powder, it comprises the following steps:
[0050] (1) Weigh 0.1997g copper acetate hydrate (Cu(CH 3 COO) 2 ·H 2 O, purity ≥99.0%), 0.4982g of copper acetate hydrate (Co(CH 3 COO) 2 4H 2 O, purity ≥99.5%) and 0.4846g of L-cysteine (C 3 h 7 NO 2 S, purity ≥ 98.5%), dissolved in 60ml of ethylene glycol, stirred to form a solution;
[0051] (2) Transfer the solution obtained in step (1) to a 100ml stainless steel reaction kettle lined with polytetrafluoroethylene, and react at 180°C for 20 hours;
[0052] (3) The product obtained in step (2) was washed with absolute ethanol and centrifuged for three times, and then dried under vacuum at 80°C for 12 hours to obtain black copper-cobalt-sulfur ultrafine powder.
[0053] As attached to the manual Figure 9 As shown, the copper-cobalt-sulfur ultrafine powder prepared in this example has the morphology of nanoparticle stacking.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com