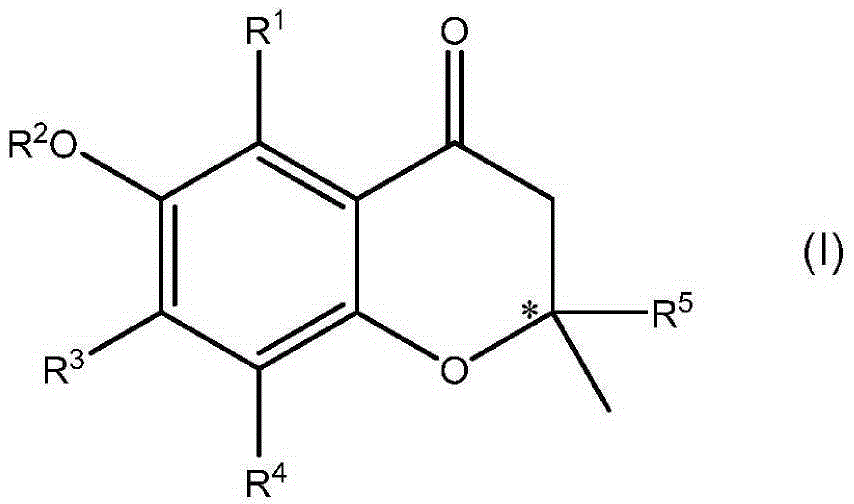
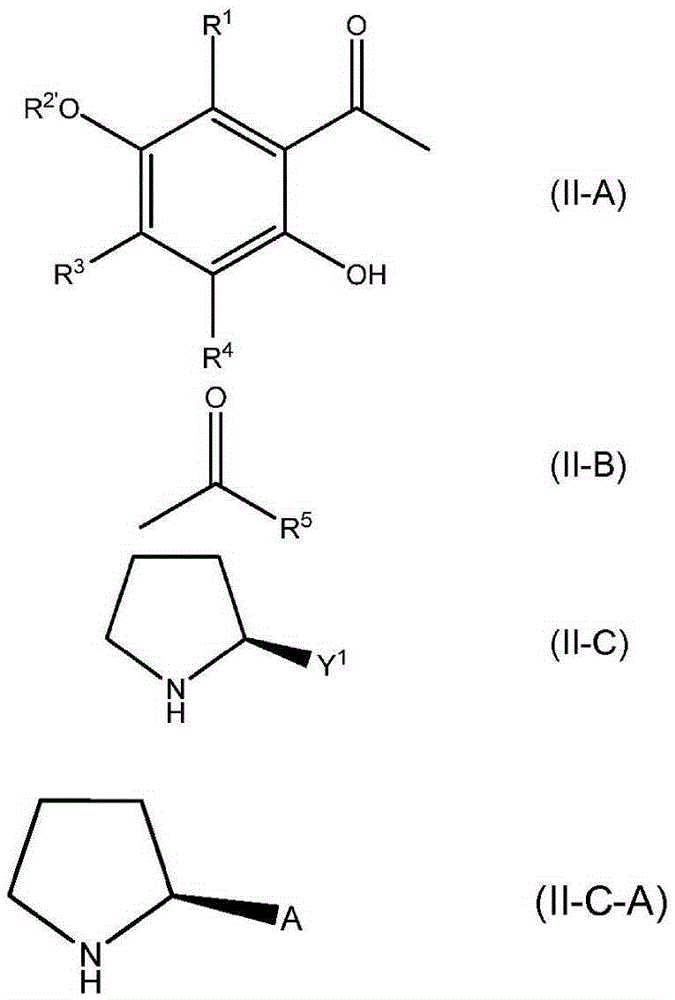
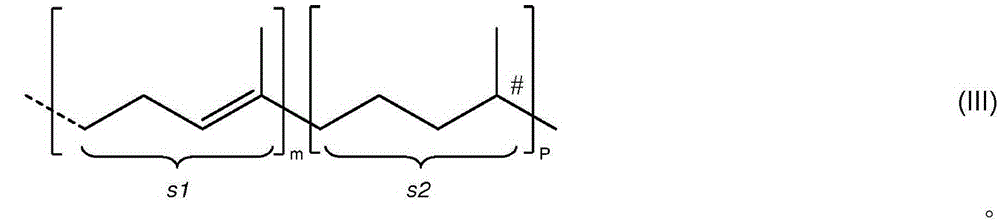
Formation of chiral 4-chromanones using chiral pyrrolidines in the presence of acids
A technology of chiral centers and chiral compounds, applied in the field of synthesis of tocopherols and tocotrienols
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0201] The present invention is further illustrated by the following experiments.
[0202] use a different acid
[0203] At 23 °C, in a 20 mL round bottom flask equipped with a magnetic stir bar, heating device, water separator and argon supply, 0.5 mmol 2-acetyl-3,5,6-trimethylhydroquinone and 0.795 mmol The acids shown in Table 1 were suspended in 2.5 mL (23.5 mmol) toluene. Then, 0.514 mmol of E,E-farnesylacetone was added, and finally 0.795 mmol of (S)-2-(methoxymethyl)pyrrolidine was added. The reaction mixture was then stirred at the temperature indicated in Table 1 for the time indicated in Table 1. When heated to 120°C, water was distilled off and the reaction mixture turned brown. After the indicated time, the reaction mixture was cooled to 23 °C. Then 1 mL of 2N HCl was added and the mixture was transferred to a separatory funnel and shaken well. The toluene phase was separated and washed with several 10 mL portions of water until a neutral aqueous phase was o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com