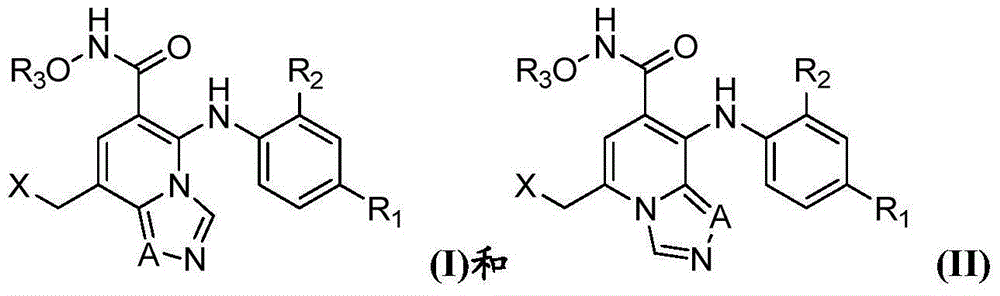
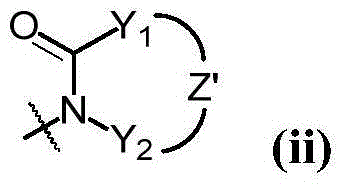
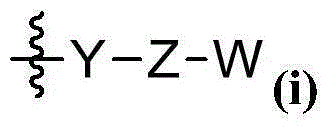Heterocyclic compound as protein kinase inhibitor and preparation method and application thereof
A compound and solvate technology, applied in the field of heterocyclic compounds as protein kinase inhibitors and their preparation and application, can solve the problems of uncontrolled cell growth, loss of cell growth, cancer, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0147] Example 1.5-(2-fluoro-4-iodophenylamino)-N-(2-hydroxyethoxy)-8-((3-oxomorpholine)methyl)imidazo[1,5-a ] Synthesis of pyridine-6-carboxamide:
[0148]
[0149] Step 1: Synthesis of 3,5-dichloro-6-methyl-2H-1,4-oxazin-2-one
[0150]
[0151] The title compound was prepared according to European Journal of Organic Chemistry; nb, 30; (2007); p.4995-4998. l H-NMR (400MHz, DMSO-d 6 )δ2.30(s,3H).
[0152] Step 2: Synthesis of ethyl 2,6-dichloro-5-methylnicotinate
[0153]
[0154] The title compound was prepared according to synthesis; nb, 9; (1991); p.765-768.
[0155] Step 3: Synthesis of ethyl 6-chloro-2-(2-fluoro-4-iodophenylamino)-5-methylnicotinate
[0156]
[0157]The compound 2-fluoro-4-iodoaniline (12.15g, 51.26mmol) was added to the reaction flask, anhydrous THF (50ml) was added, and after cooling to -78°C under a nitrogen atmosphere, LiHMDS was added dropwise to the reaction solution (1M, 128.5ml, 128.5mmol), after reacting at -78°C for 30 minutes,...
Embodiment 2
[0184] Example 2: N-(2,3-hydroxypropoxy)-5-(2-fluoro-4-iodophenylamino)-8-((3-oxomorpholine)methyl)imidazo[1 ,5-a]Synthesis of pyridine-6-carboxamide
[0185]
[0186] Step 1: Ethyl 5-(2-fluoro-4-iodophenylamino)-8-((3-oxomorpholine)methyl)imidazo[1,5-a]pyridine-6-carboxylate synthesis
[0187]
[0188] The compound 5-(2-fluoro-4-iodophenylamino)-8-((4-(methylamino)-4-oxobutoxyamino)methyl)imidazo[1,5-a] Add ethyl pyridine-6-carboxylate (500mg, 0.88mmol) into the reaction flask, add 1,2-dichloroethane (10ml), heat to 60°C and react for 10 hours, after the reaction is completed, cool to room temperature, Concentrate and separate the product by column chromatography. (400mg, yield: 84.6%). [M+H] + =539.3
[0189] Step 2: N-((2,2-Dimethyl-1,3-dioxolan-4-yl)methoxy)-5-(2-fluoro-4-iodophenylamino)-8- Synthesis of ((3-oxomorpholine)methyl)imidazo[1,5-a]pyridine-6-carboxamide:
[0190]
[0191]Compound O-((2,2-dimethyl-1,3-dioxolan-4-yl)methyl)hydroxylamine (41mg, 0....
Embodiment 3
[0195] Example 3: N-(cyclopropylmethyloxy)-5-(2-fluoro-4-iodophenylamino)-8-((3-oxomorpholine)methyl)imidazo[1,5- a] the synthesis of pyridine-6-carboxamide:
[0196]
[0197] Compound O-(cyclopropylmethyl)hydroxylamine (12mg, 0.14mmol) was added to the reaction flask, anhydrous THF (1ml) was added, and after cooling to -78°C under a nitrogen atmosphere, LiHMDS was added dropwise to the reaction solution (1M, 0.27ml, 0.27mmol), after reacting at -78°C for 30 minutes, add the compound 5-(2-fluoro-4-iodophenylamino)-8-((3-oxomorpholine)methyl) Ethyl imidazo[1,5-a]pyridine-6-carboxylate (50mg, 0.09mmol), after the addition, the dry ice was removed, and the reaction was continued for 3 hours. After the reaction was completed, the reaction was quenched with saturated ammonium chloride, extracted with ethyl acetate, dried over anhydrous sodium sulfate, concentrated, and separated by column chromatography to obtain the product. (50 mg, yield: 55%). 1 HNMR (400MHz, CD 3 OD) 1 ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap