Clindamycin hydrochloride cream and preparation method thereof
A technology for clindamycin hydrochloride and cream, which is applied in ointment delivery, pharmaceutical formula, emulsion delivery, etc. It can solve problems such as degradation, easy oxidation, and excessive growth of impurities, so as to achieve stable quality and inhibit the hydrolysis process , to avoid the effect of the hydrolysis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
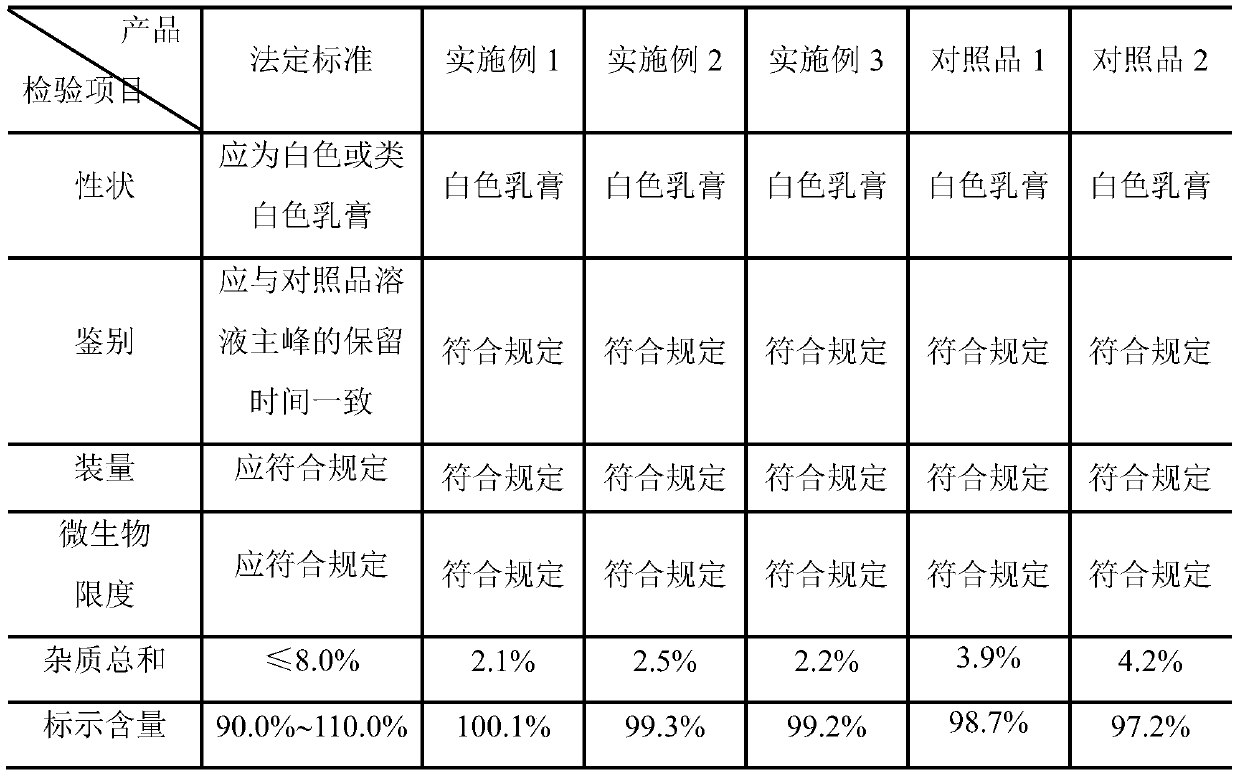
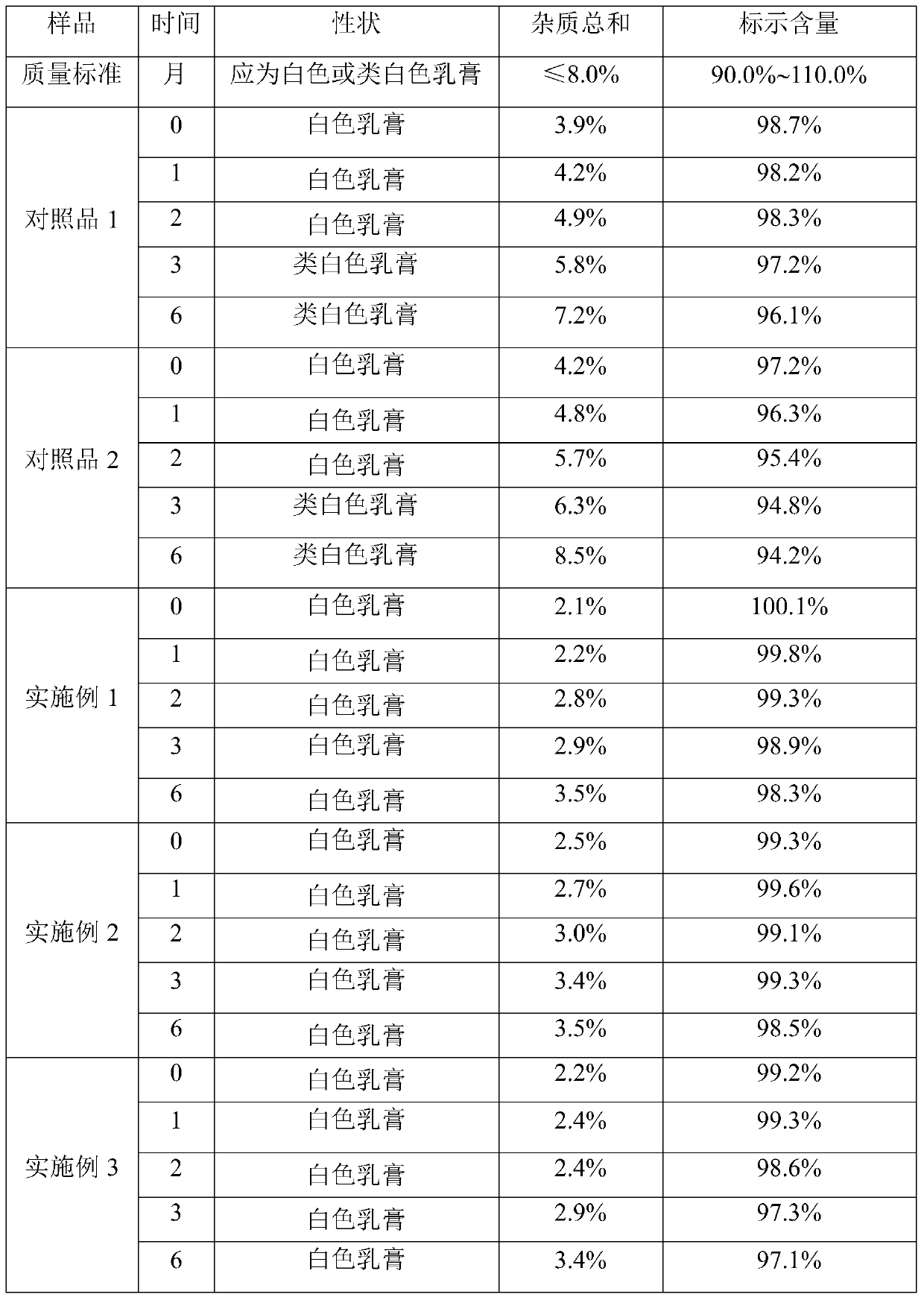
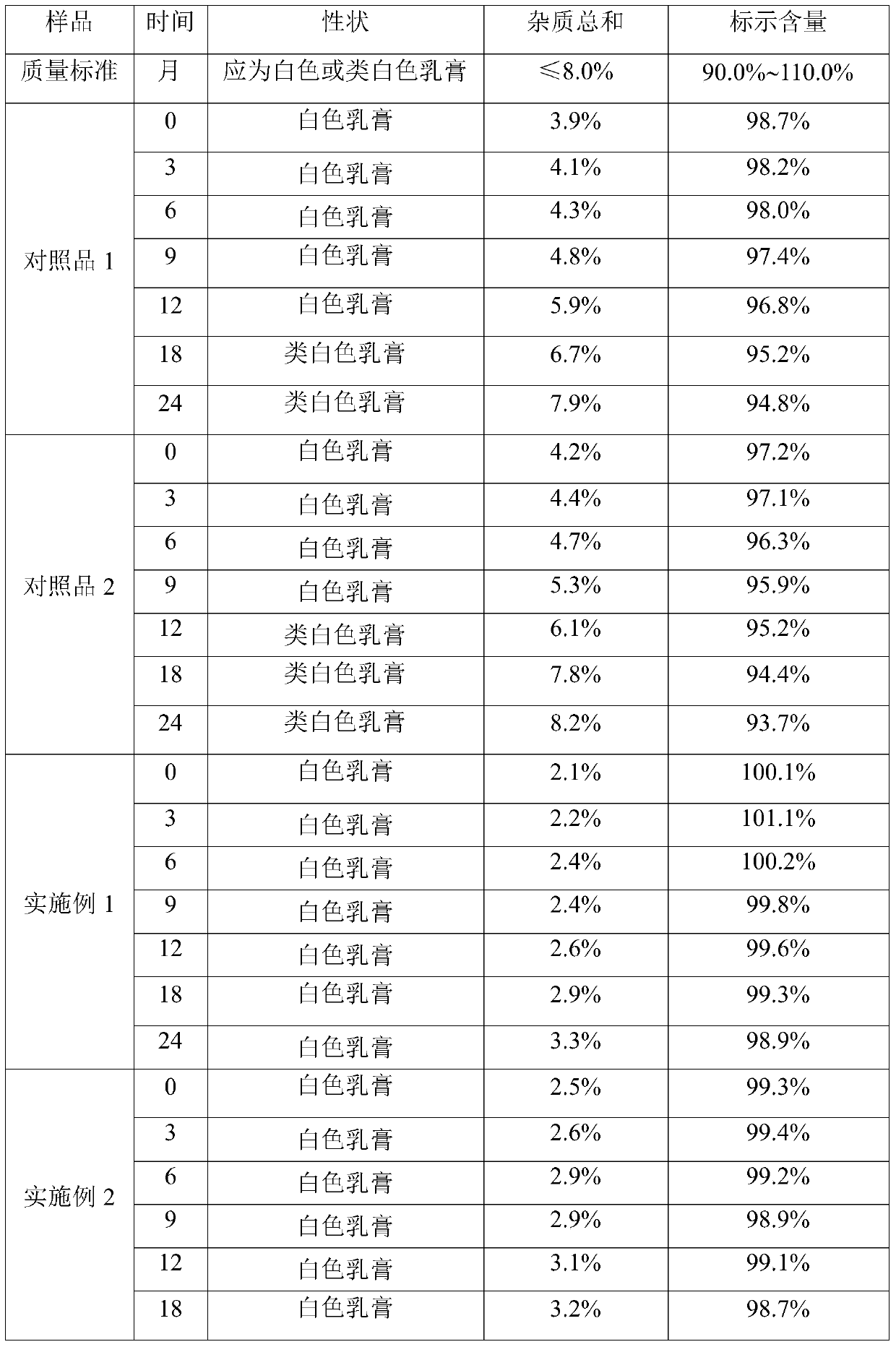
[0026] A kind of clindamycin hydrochloride cream, in every 10g clindamycin hydrochloride cream: the weight that contains clindamycin hydrochloride is 0.1g, cetyl alcohol is 0.7g, light liquid paraffin is 1.4g, white vaseline 0.8g of propylene glycol, 0.5g of propylene glycol, 0.8g of Span 60, 5mg of potassium sorbate, 5mg of ethylparaben, 10mg of 2,6-di-tert-butyl p-cresol, 4mg of Pechoin essence, and the rest 10% phosphoric acid solution and purified water.
[0027] The preparation process of the clindamycin hydrochloride cream is as follows: add white petrolatum, the oil phase matrix, into the oil phase pot, heat to 80°C-90°C to melt, add clindamycin hydrochloride to the melted In the white vaseline, continue to add cetyl alcohol, light liquid paraffin and Span 60, 2,6-di-tert-butyl-p-cresol, potassium sorbate and ethylparaben, continue to stir and melt to obtain the oil phase; Stir Pechoin essence, propylene glycol and purified water evenly, use 10% phosphoric acid solutio...
Embodiment 2
[0029] A kind of clindamycin hydrochloride cream, in every 10g clindamycin hydrochloride cream: the weight that contains clindamycin hydrochloride is 0.05g, cetyl alcohol is 1.0g, light liquid paraffin is 1.0g, white vaseline 1.0g for propylene glycol, 0.8g for propylene glycol, 0.6g for Span 60, 2mg for potassium sorbate, 2mg for ethylparaben, 10mg for 2,6-di-tert-butyl p-cresol, 4mg for strawberry essence, and the rest 20% citric acid solution and purified water.
[0030] The preparation process of the clindamycin hydrochloride cream is as follows: add white petrolatum, the oil phase matrix, into the oil phase pot, heat to 80°C-90°C to melt, add clindamycin hydrochloride to the melted In the white vaseline, continue to add cetyl alcohol, light liquid paraffin and Span 60, 2,6-di-tert-butyl-p-cresol, potassium sorbate and ethylparaben, continue to stir and melt to obtain the oil phase; Stir strawberry essence, propylene glycol and purified water evenly, use 20% citric acid s...
Embodiment 3
[0032] A kind of clindamycin hydrochloride cream, in every 10g clindamycin hydrochloride cream: the weight that contains clindamycin hydrochloride is 0.2g, cetyl alcohol is 0.5g, light liquid paraffin is 2.0g, white vaseline 0.5g of propylene glycol, 0.6g of propylene glycol, 1.5g of Span 60, 10mg of potassium sorbate, 10mg of ethyl paraben, 5mg of 2,6-di-tert-butyl p-cresol, 2mg of strawberry essence, and the rest 15% phosphoric acid solution and purified water.
[0033] The preparation process of the clindamycin hydrochloride cream is as follows: add white petrolatum, the oil phase matrix, into the oil phase pot, heat to 80°C-90°C to melt, add clindamycin hydrochloride to the melted In the white vaseline, continue to add cetyl alcohol, light liquid paraffin and Span 60, 2,6-di-tert-butyl-p-cresol, potassium sorbate and ethylparaben, continue to stir and melt to obtain the oil phase; Stir strawberry essence propylene glycol and purified water evenly, use 20% citric acid solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com