Sobetirome in the treatment of myelination diseases
A disease and myelin technology, which is applied in the field of sobuterosol for the treatment of myelination diseases, which can solve the problems of no effective treatment and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
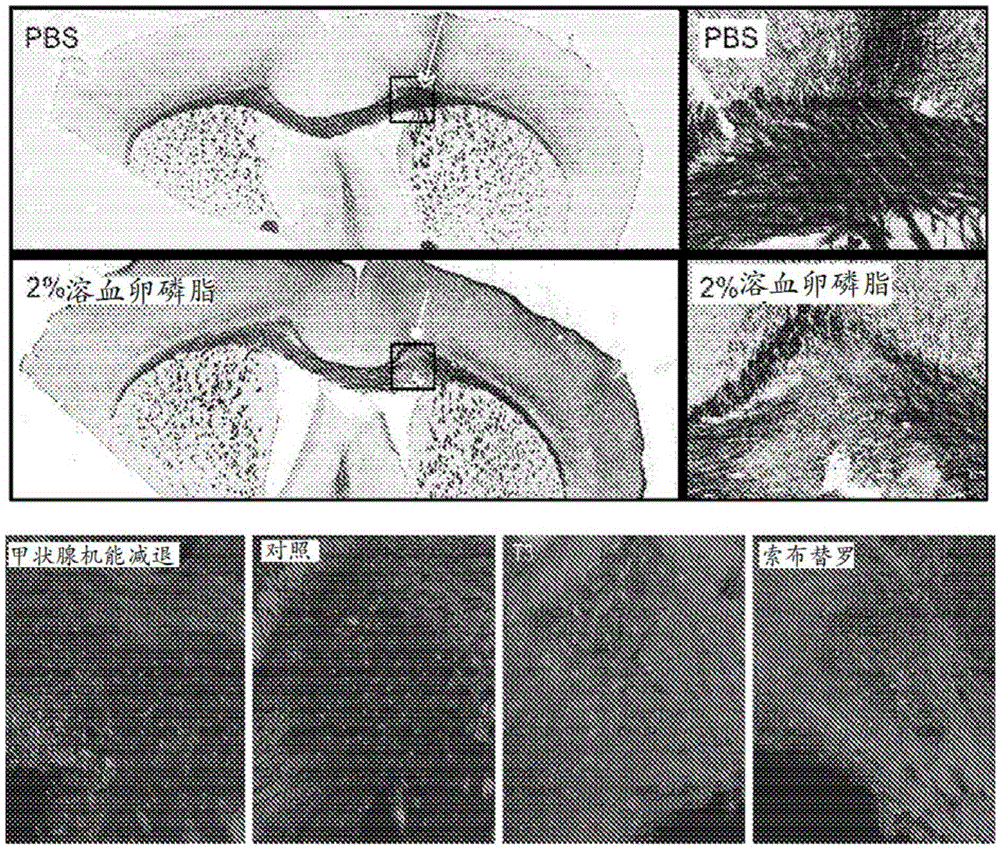
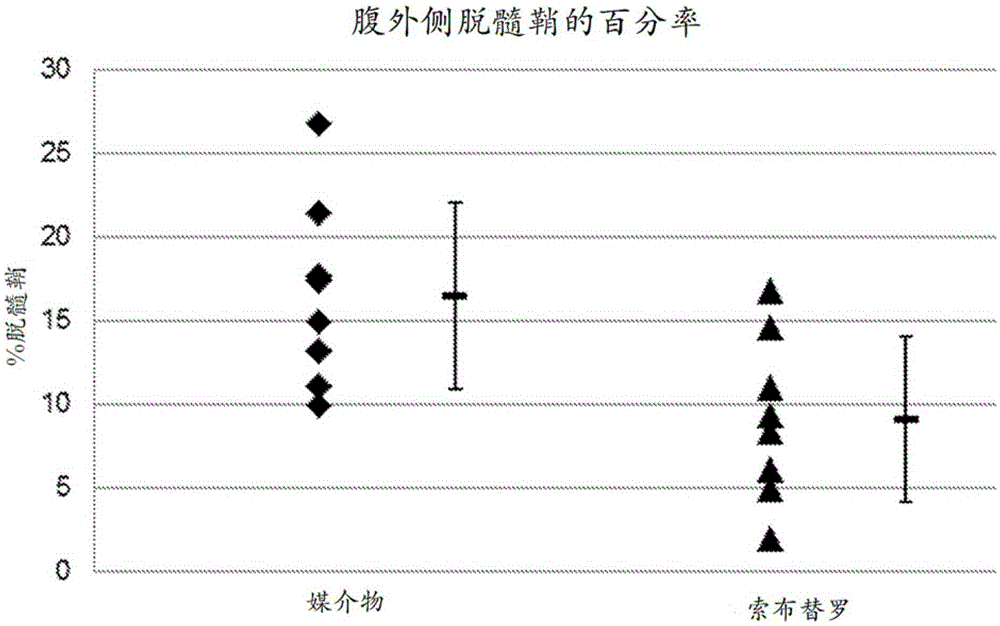
[0137] Embodiment 1: Sobutirol is used for the purposes of treating multiple sclerosis (MS)
[0138] This example describes the discovery that treatment with sobutirol reduces demyelination in two different animal models of MS.
[0139] Chronic demyelination contributes to disability and progressive damage in MS
[0140] In MS, inflammatory cells induce multifocal demyelination and variable axonal degeneration in the CNS, termed MS plaques. As a result, individuals with MS develop a variety of neurological deficits, including paralysis, gait impairment, cognitive impairment, sensory loss, and decreased vision. Although remyelination occurs spontaneously in MS as part of the natural repair process, it is incomplete and tends to become ineffective as the disease progresses. Failure of remyelination results in chronically demyelinated axons that lose their ability to normally conduct axons, leading to neurological dysfunction. Importantly, chronic demyelination contributes to ...
Embodiment 2
[0153] Example 2: Sobutirol in an animal model of neonatal hypoxia
[0154] Chronic neonatal hypoxia is a clinically relevant model of preterm brain injury caused by insufficient gas exchange due to lung hypoplasia. This hypoxic state is a significant contributor to the diffuse white matter injury (DWMI) commonly seen in preterm infants. Chronic hypoxia can cause abnormal myelination. A mouse model of chronic hypoxia has been described previously (Scafidi et al., Nature doi: 10.1038 / naturel2880 [epub ahead of publication], 5 December 2013). This model can be used to evaluate the effect of sobutirol on oligodendrocyte regeneration and remyelination after hypoxia.
[0155] Mice were randomly selected to undergo hypoxic feeding or serve as normoxic controls. Hypoxic mice were placed in a sealed chamber, as previously described, by dosing with N 2 Replace O 2 The concentration was maintained at 10.5% (Raymond et al., JNeurosci 31:17864-17871, 2011; Bi et al., JNeurosci 31:920...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com