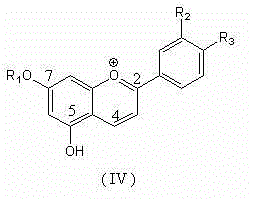
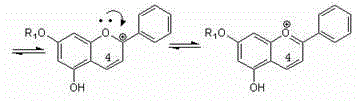
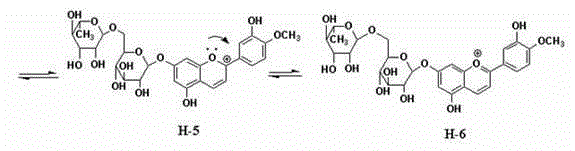
Method for semi-synthesizing 3-deoxyanthocyanidin from 5-OH-7-O-substituted flavanone
A 5-OH, dihydroflavonoid technology, used in the fields of food, cosmetics, chemistry and medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Take 20g of naringin with 98% naringin in 50ml of DMSO and heat to dissolve. Take another 3g of KBH4 and add in portions until naringin is completely reduced to naringin-type flavan-4-ol (about overnight, try to prevent absorption of moisture in the air, UV monitoring , scanning under alkaline conditions, there is no absorption peak at 360nm). Slowly add 5ml of concentrated sulfuric acid and stir evenly (the solution turns light red, generating naringin-type flavan-4-cations, taking a small amount of water and diluting, the color can fade, and generating naringin-type flavan-4-ol ), slowly added 120ml of anhydrous n-butanol, precipitated to the bottom, stood still for 30min, poured out the n-butanol solution, added 20ml of anhydrous n-butanol to the precipitate, stirred quickly to dissolve, poured out the n-butanol, and 40ml of anhydrous pyridine was added, 8.7g of iodine was added, stirred until dissolved, and the reaction was continued in a water bath at 55°C for 96h ...
Embodiment 2
[0085] Take 5g of the Apigeninidin-7-O-neohesperidoside sample in Example 1, add 100ml of 10% hydrochloric acid and 20% ethanol solution to hydrolyze at 60°C for 10h, add 100ml of water, wash with 120g of macroporous adsorption resin D101, 500ml of water to remove impurities, and wash with 1500ml of 75% ethanol After decompression, the ethanol was recovered under vacuum to obtain Apigeninidin, 1.7g of orange powder. [M] + 255; a small amount dissolved in water is orange, and paper chromatography shows yellow spots; on the UV spectrum, there are maximum absorption at 480nm and 279nm; the color and UV spectrum are consistent with the literature (P.SuganyaDevi1, M.Saravanakumar, S.Mohanda. Identification of 3-deoxyanthocyanins from redsorghum (Sorghumbicolor) bran and its biological properties. African Journal of Pure and Applied Chemistry, 2011, 5(7):181-193).
[0086] Example 3: Preparation of Diosmetinidin-7-O-rutinoside from Hesperidin
Embodiment 3
[0087]Take 20g of hesperidin with a content of 95% (pre-dried at 90°C for 3-4 hours), heat and dissolve 50ml of DMSO, and take another 3g of KBH4, and add in portions until the naringin is completely reduced to hesperidin-type flavan 4-ol (about overnight , UV monitoring, scanning under alkaline conditions, no absorption peak at 360nm). Slowly add 5ml of concentrated sulfuric acid (first 2ml is very slow, the solution turns light red), stir evenly (the solution turns light red), add slowly add 120ml of anhydrous n-amyl alcohol, precipitate and sink to the bottom, stand still for 30min, pour out the normal Pentanol solution, add 20ml of anhydrous n-pentanol to the precipitate, quickly stir and knead to dissolve, pour out the n-pentanol solution, and treat it twice in the same way, add 40ml of anhydrous pyridine, add 8.3g of iodine, stir until dissolved, add anhydrous 5g of calcium sulfate in water, stirred evenly, and reacted continuously in a water bath at 55°C for 96h under a...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap